This page was produced as an
assignment for an undergraduate course at Davidson College.
What
is a chemokine receptor?
First it is important to know what a chemokine is. Chemokines act as attractant molecules for effector cells in the immune system. They can be released by many types of cells, and used to attract cells in the innate response system as well as the adaptive response system. Chemokines fall into two broad groups called CC chemokines and CXC chemokines, classified according to the position of the location of the cysteine residues near the amino terminus. In CC chemokines, the cysteines are side by side, whereas another amino acid separates the cysteines in the CXC chemokines. Clearly, CC chemokines bind to CC receptors. CCR3 is a CC receptor, as can easily be recognized by its name. (Janeway et al. 2001)
Specific characteristics of CCR3
CCR3 can serve as a receptor for several different chemokines. Some of these include: MIP-1α, MIP-1β, RANTES, monocyte chemoattractant protein-2, 3, and 4, and eotaxin 1, 2, and 3. (Ma et al. 2002, Humbles et al. 2002, Janeway et al. 2001, BioCarta 2002) MIP-1α and MIP-1β compete with HIV-1, in addition to other things. RANTES also has a role in HIV-1. Eotaxin is involved in allergy response. All but eotaxin can also bind to CCR1 and CCR5, suggesting a similarity in the sequences and folding of the receptor proteins. (Janeway et al. 2001) Both in vivo and in vitro, eotaxin and RANTES have been shown to stimulate migration of eosinophils in humans as well as other animals. Most of the ligands to CCR3 are associated with asthma, and CCR3 has become an appealing possibility in asthma treatment or therapy. (Ma et al. 2002, Humbles et al. 2002)
Eotaxin has received a lot of attention in the study of the effects of CCR3 in asthma and allergies. It is a β chemokine, produced by monocytes and T cells, and in the endothelium and epithelium. (Harrington et al. 1999, Janeway et al. 2001) It mainly activates eosinophils to a site of inflammation, but can also act to stimulate monocytes and T cells. (Harrington et al. 1999, Janeway et al. 2001, BioCarta 2002) Eotaxin has an important role in directing eosinophil accumulation to allergy airways, and it has been used in experiments to determine eosinophil attraction to certain organs and patterns of migration. (Harrington et al. 1999, Humbles et al. 2002, Williams 2002) In addition, eotaxin regulates eosinophil levels in the blood. (Williams 2002) Eosinophils are leukocytes involved in inflammatory response and defense against parasites. (BioCarta 2002, Janeway et al. 2001)
All chemokine receptors are integral membrane proteins that are also coupled to G-proteins. That is, when the ligand binds to the chemokine receptor, it sets off a chain of reactions that begin with the G-protein. (See diagram below.) CCR3 is a seven-helix transmembrane protein, and it is suggested that the binding site consists of a complex of several domains. (Efremov et al. 1999, Ma et al. 2002, Pease 2002) This β chemokine receptor can be found on eosinophils, subsets of TH2 cells, basophils, mast cells, neural tissue, and some epithelia cells. (Humbles et al. 2002, Tang and Powell 2001) The first four cells t ypes are crucial in allergy response. (Humbles et al. 2002) Approaches in targeting events further along in the allergic inflammatory cascade, including CCR3, are currently being investigated. (Tang and Powell 2001) CCR3 is important in the recruitment of eosinophils at basal levels to the mucosal tissues of the gastrointestinal tract, and the lung. (Humbles et al. 2002, Ma et al. 2002) A role for CCR3 in mast cell homing has also been detected. (Humbles et al. 2002)
The
CCR3 gene is located on chromosome 3.
Click here
for some additional technical information on CCR3 from NCBI.
Fun pictures of CCR3
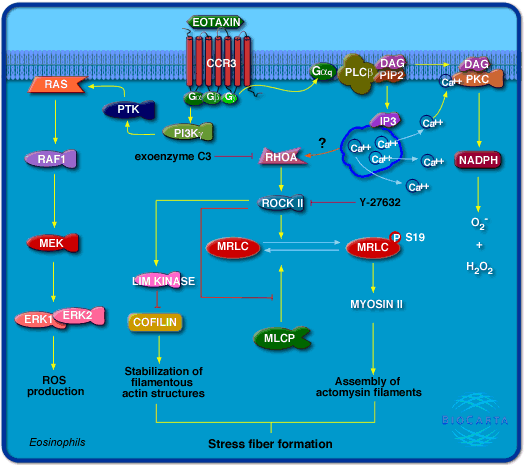
Figure 1: Diagram of what pathways can be activated by eotaxin binding to CCR3 on eosinophils in order to induce inflammatory response. For a full description of what’s going on, click on the diagram. Image reproduced with permission from BioCarta.
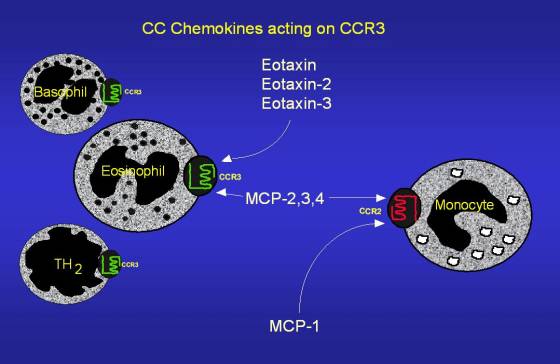
Figure 2: Example of chemokines that act on CCR3. The diagram correctly shows the seven transmembrane section of CCR3, as well as an intracellular and extracellular domain. Image reproduced with permission. (Conroy 2002) Click on the diagram to be directed to the original site.
Figure 3: Structural image of CCR1. CCR3 has been shown to be most similar in structure to CCR1. Image reproduced with permission. (Pease 2002) Click on the figure to be directed to the original site.
How did the scientists find all of this out?
How do scientists find anything out? They do experiments! A few key experiments and their findings are briefly described below.
1) Efremov et al. performed an experiment that confirmed common orientations in the first extracellular loops of CCR3, CCR5, and CCR2B when compared to other human G-proteins. Different chimeras determined that the first extracellular loop of CCR5, as well as the amino terminus to a lesser extent, was essential to coreceptor function for the human immunodeficiency virus. Identification of the similarity in these proteins suggests roles for CCR3 and CCR2B in HIV entry. These receptors differ from each other in primary (amino acid) structure, but the conformation and polarity of the binding site seem to be more important. Some receptors have similar structures (mCCR5, for example) but have not been reported to serve as coreceptors for HIV-1. The polarity of the side chain on mCCR5 is different than that of the three receptors mentioned previously. (Efremov et al. 1999)
2) Humbles et al. found several significant occurrences in the disruption of the CCR3 gene in mice.
A. CCR3 deficient mice had a seven-fold reduction in the number of eosinophils in the small intestine and a 6-fold increase in the spleen. Numbers in the lung and thymus remained unchanged. This confirmed that CCR3 was important in keeping the basal levels of eosinophils in the gastrointestinal tract, but not the lung. (No allergen challenge; and I assume ‘allergen challenge’ refers to introducing allergens to the system)
B. After allergen challenge in CCR3-/- mice, there was a 50-70% reduction in eosinophil numbers, but an increase in the spleen, suggesting that eosinophils not directed to the lung go to the spleen. Most of the eosinophils in the lung tissue were caught in blood vessels in the subendothelial space. This discovery suggests CCR3 (or the eotaxin/CCR3 complex) is necessary for final extravasation across the membrane into the tissue.
C. In the absence of allergen challenge, both wild type and CCR3-/- mice show little response to inhalation of methacholine. With allergen challenge, CCR3 deficient mice show increased airway hyperresponsiveness. The gene disruption intensifies airway hyperresponsiveness to methacholine, even in the presence of extra eosinophils to the lungs.
D. After allergen challenge, there were increased numbers of mast cells in the trachea of the knockout mice. An increase of mast cells was not observed in the small intestine, spleen, or skin.
E. Early data show that if there are no intraepithelial cells present, CCR3-/- mice are protected completely from allergen-induced airway hyperresponsiveness. (Humbles et al. 2002)
3) Other research backs up Humbles et al.: “The eosinophil response in inflammation is absent in mice lacking CCR3, indicating the key role of this G protein coupled receptor in inflammation and allergic responses.” (BioCarta 2002)
4) Ma et al. conducted an experiment with a very similar name to the one conducted by Humbles et al. 2002. So much so, that I had to read the titles several times in order to make sure I wasn’t using the same article. Anyway, however similar the titles might be, the experiments are different and so came to different conclusions.
A. In sham-sensitized (?) wild type mice, few eosinophils were present. This number increased following ovalbumin sensitization. In CCR3-/- mice, no eosinophils were detected in sham-sensitized skin and were “virtually absent” after ovalbumin sensitization. These data show that there are few, if any, eosinophils present on the skin of CCR3-/- mice, and numbers do not increase as expected after ovalbumin sensitization. However, basal eosinophils numbers remained constant in the lung and thymus, and eosinophils from CCR3-/- mice migrating normally when transplanted into the trachea of wild type mice, signifying that CCR3 is critical for eosinophil recruitment to the lung and the skin. Humbles et al. had concluded that eosinophils had other non-CCR3 dependent methods for recruitment to allergic lungs. This data is contradictory, but I think it is just an issue of when the experiments were conducted. Dr. Humbles was a part of the Ma team a month after her experiment.
B. CCR3 is expressed on TH2 cells but not TH1 cells. In both wild type and CCR3-/- mice sensitized with different allergens, the TH2 levels were equal. This implies that CCR3 is possibly not important for TH2 differentiation, nor is the receptor involved in TH2 recruitment to inflammation sites.
C. The team also found that mast cell recruitment into the epithelium of the airway by a CCR3-independent process is an important factor in airway hyperresponsiveness under a specific model. In another model, a CCR3-dependency of eosinophil recruitment is key in the absence of mast cells in the epithelium cells of the airway. (The differing models are due to two separate sensitization methods.) (Ma et al. 2002)
5) The paper by Vassiliadis et al. mainly compared the role of interleukin-1β (IL-1β) on CCR5 and CCR3. These receptors were shown to be expressed on pancreatic insulin-producing model system cells.
A. Expression of both on the cell surface was decreased in the presence of IL-1β. However, CCR3 has been shown to be down regulated by IL-3, and CCR5 by RANTES and IL-10. On eosinophils, CCR3 was held inside the cell by aminooxypentane-RANTES. This event is similar to the one shown by the Vassiliadis team. Nonetheless, IL-1β was able to retain CCR5 and CCR3 in the cell cytoplasm. A decrease in expression of CCRs could mean protection against the autoimmune disease insulin-dependent diabetes mellitus, which destroys insulin-secreting cells in the pancreas.
B. In the presence of IL-1β, soluble CCR5 secretion is hampered, but soluble CCR3 secretion is unchanged. The receptors were not destroyed, but broken down by endosomes and reutilized. (Vassiliadis et al. 2002)
References
BioCarta, 2002. “CCR3 signaling in Eosinophils.” http://www.biocarta.com/pathfiles/h_CCR3Pathway.asp (February 27, 2003).
Conroy, D. 2002. “Role of chemokines in eosinophil recruitment to sites of inflammation.” Faculty of Medicine, Imperial College of Science. http://www.med.ic.ac.uk/divisions/3/Lb7.asp (February 28, 2003).
Efremov, R., M.J. Truong, E. Darcissac, J. Zend, O. Grau, G. Vergoten, C. Debard, A. Capron, and G. Bahr. 1999. Human chemokine receptors CCR5, CCR3 and CCR2B share common polarity motif in the first extracellular loop with other human G-protein coupled receptors: Implications for HIV-1 coreceptor function. Eur. J. Biochem. 263: 746-756
Harrington, P.M, D.J. Newton, C.M.M. Williams, J.A. Hunt, R.J. Dearman, I. Kimbers, J.W. Coleman, and B.F. Flanagan. 1999. Eotaxin and eotaxin (CCR3) expression in Sephadex particle-induced rat lung inflammation. Int. J. Exp. Path. 80: 177-185
Humbles, A., B. Lu, D. Friend, S. Okinaga, J. Lora, A. Al-garawi, T. Martin, N. Gerard, and C. Gerard. 2002. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc. Natl. Acad. Sci. USA 99(3): 1479-1484
Janeway, C., P. Travers, M. Walport, and M. Shlomchik. 2001. Immunobiology, 5th ed. Garland, NY, pp. 71-73, 691
Ma, W., P. Bryce, A. Humbles, D. Laouini, A. Yalcindag, H. Alenius, D. Friend, H. Oettgen, C. Gerard, and R. Geha. 2002. CCR3 is essential for skin eosinophilia and airway hyperresponsiveness in a murine model of allergic skin inflammation. J. Clin. Invest. 109(5): 621-628
Pease, J. 2002. “Structure Function Relationships of Chemokine Receptors.” Faculty of Medicine, Imperial College of Science. http://www.med.ic.ac.uk/divisions/3/Lb7.asp (February 28, 2003).
Tang, M.L.K. and C. Powell. 2001. Childhood asthma as an allergic disease: rationale for the development of future treatment. Eur. J. Pediatr. 160: 696-704
Vassiliadis, S., V. Balabanidou, G. Papadopoulos, and I. Athanassakis. 2002. Localization and Expression of CCR3 and CCR5 by Interleukin-1-Beta in the RIN-5AH Insulin-Producing Model System: A Protective Mechanism involving Down-Regulation of Chemokine Receptors. JOP. J Pancreas (Online) 3(3): 66-75 http://www.joplink.net/prev/200205/02.html
Williams, T. 2002. “Mechanisms Underlying Eosinophil Accumulation in the Asthmatic Lung.” Faculty of Medicine, Imperial College of Science. http://www.med.ic.ac.uk/divisions/3/Lb7.asp (February 28, 2003).
Davidson College Biology Homepage
Other Homepages by this Student
Questions, concerns or comments? Email the editor