This web page was produced as an assignment for an undergraduate
course at Davidson College.
Human DNA Helicase WRN (RecQL2)
The human DNA helicase WRN, a member of the RecQL family of helicases, unwinds double-stranded DNA in the 3'-5' direction and also possesses a 3'-5' exonuclease activity. This protein is thought to be involved in DNA repair and stability mechanisms. Mutations of the WRN gene result in Werner's syndrome, a premature aging disease. (OMIM, 2003).
DNA Helicases- An Overview
DNA helicases bind to DNA strands and, through conformational changes caused by ATP hydrolysis, break the hydrogen bonds between the bases of double-stranded DNA. This activity gives helicases an important role in many cellular processes including genome replication, recombination, repair, and transcription (Kitao et. al.¸1998).

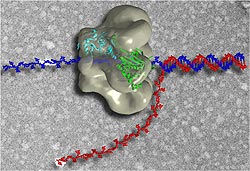
Figure 1. The Activity of a DNA Helicase. This image shows an artist's representation of the activity of a T7 DNA helicase. The basic process of unwinding the DNA double helix is common to all DNA helicases across species. Image from http://www2.umdnj.edu/bchemweb/HTML/newHandbook.html (courtesty Dr. Smita Patel and Dr. Edward Egelman) Permission Obtained.
Helicase WRN (RecQ2)
Structure
The gene which encodes the WRN protein has been located at the gene map locus 8p12-p11.2 on the human chromosome 8 (OMIM, 2003). The transcript of the gene is 140.5 kb long with many introns and exons. The helicase protein produced from this gene consists of 1432 residues and has an overall charge of -5. (Sanger Institute- Ensembl Protein Report, 2003). The WRN helicase consists of several major structural domains (Figure 2).
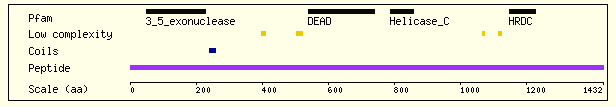
Figure 2. The structural/functional domains of WRN. This diagram shows some of the major structural domains of the WRN protein that are important to its function. It also indicates the locations of coils and regions of low complexity and their relative positions in the amino acid sequence (N-terminal to C-terminal shown left to right). Image from http://www.ensembl.org/Homo_sapiens/protview?peptide=ENSP00000298139&db=core Permission Obtained.
The 3'-5' exonuclease region of the protein is 171 amino acids long and is located at the N-terminal end of the protein. It is highly similar to the exonuclease regions of proteins such as DNA polymerase that have DNA proofreading activity. When exposed to double stranded DNA, these residues form a hexamer around the end of the strand and remove bases toward the 5' end. This region is essential to the DNA repair function of WRN (Xue et. al., 2001).
The DEAD box domain of the protein is highly conserved among DNA and RNA helicases. The central feature, as the name of the domain implies, is a sequence of amino acids Asp-Glu-Ala-Asp (this may be varied as DExH/D in other helicase proteins). Along with the helicase-C (carboxy-terminal helicase) domain, this region of the protein is thought to be the essential to the helicase activity of the protein (Sanger Institute- Ensembl Protein Report, 2003).
The HRDC domain consists of 80 amino acids at the C-terminal end of the protein. It contains five helices connected by short turns or hydrophobic loops. Certain hydrophobic residues within the loops are necessary for the packing of this domain into a bundle. A patch near helix a4 (see Fig 3) has been shown to interact with DNA in a non-sequence specific manner, suggesting that this domain may contribute to the interaction between the WRN protein and the double stranded DNA as it performs its helicase function. (Liu et. al., 1999)
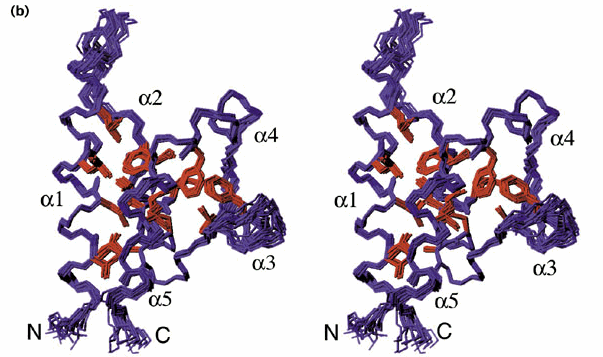
Figure 3. The structure of the HRDC domain. The structure of the HRDC domain shown in this diagram was determined by NMR spectroscopy from the WRN homolog Sgs1. The residues shown in red are the highly conserved hydrophobic sidechains that are essential to the folding of the protein. (Liu et. al. 1999). Image from http://journals.bmn.com/journals/list/browse?uid=JSTR.st7c05&rendertype=text Permission Pending.
In addition to these regions, the WRN protein, like other helicases, has two conserved ATP binding sites (labeled A and B) which also contain locations for the association of Mg cations. The binding and hydrolysis of ATP by these regions is dependent on magnesium binding and provides the energy necessary for the DNA helicase to break the hydrogen bonds between the bases of the double stranded DNA and unwind the helix (Puranam, 1994).
Function
Like all helicases, the basic activity of WRN is the unwinding of the DNA double-helix using the energy of ATP hydrolysis. WRN is a 3’-5’ helicase (unwinds the double stranded DNA in the 3’ to 5’ direction) (Mohaghegh et al, 2001). This directionality indicates that the helicase cannot be involved in processes like transcription and replication that occur in the 5’ to 3’ direction, so this helicase must be involved in other functions.
Though the function of this protein has not been definitively determined, studies indicate that WRN is involved in DNA repair mechanisms and the promotion of genome stability. Over the lifespan of a cell, genomic DNA can accumulate damage from external shocks or replication errors. If these errors are not corrected, intracellular signaling pathways will trigger apoptosis (programmed cell death) (Alberts et. al., 2002). WRN is thought to provide the unwinding of the DNA double helix and some of the exonuclease function necessary to allow the repair of DNA damage. This function is supported by the knowledge that a closely related protein, E. coli RecQ, is also involved in DNA repair mechanisms (Puranam, 1994).
Some research suggests that the WRN protein performs this DNA repair when problems occur at the replication fork that prevent the progression of replication. This may occur when the helicase associates with a topoisomerase during DNA replication or recombination. The topoisomerase protein cuts the DNA at regular intervals as it is unwound, allowing it to unwind smoothly and without becoming tightly coiled at the end of the strand. The helicase may associate with this protein to correct mistakes which are made in this cutting process and to allow smooth progression of the replication fork, ensuring efficient recombination or replication (Chakraverty et. al., 1999).
Location
Whereas other human helicases are located throughout the cells of the human body, WRN appears to be somewhat tissue-specific. Northern blot analyses of the expression of this protein have indicated that it is expressed at low levels in many tissues, but preferentially in the pancreas and testis. These data suggest that while the DNA repair function performed by WRN is necessary to cell vitality, it may be performed predominantly by other proteins (perhaps homologs of WRN) in some tissues (Kitao, 1998).
Within cells, immunofluorescence shows that WRN is located in the nucleus and
nucleolar regions. This is consistent with the function of the protein in maintaining
the integrity of the genetic material in the nucleus (OMIM, 2003).
Human Disease Caused by Mutation of WRN- Werner's Syndrome
Eight different mutations in the WRN gene lead to the disease known as Werner's Syndrome. Though an individual suffering from this disease will tend to develop normally as a child, rapid and premature aging occurs after puberty (Fig 4). Defects of the skin and a propensity for the development of premature arteriosclerosis and diabetes mellitus are symptoms related to this early aging process. Cells of Werner's syndrome patients undergo senescence (cell death) much sooner than normal cells (OMIM, 2003).
The mutations in the WRN gene that lead to Werner's syndrome typically occur in the HRDC domain mentioned earlier. Since this domain is predicted to be active in enabling the DNA association of this helicase, a mutation in the region likely prevents the protein's activity in DNA repair mechanisms (Liu et. al., 1999). As mentioned, if the DNA of a cell accumulates damage, it will undergo apoptosis. Thus, the loss of the WRN protein function leads to early cell death and thus premature aging of the individual.
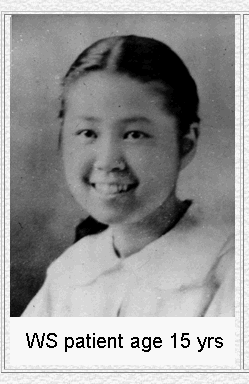
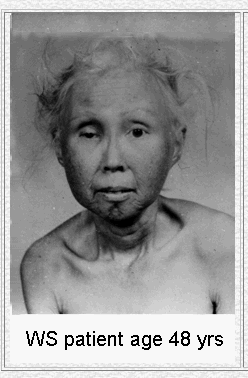
Figure 4. The premature aging effects of Werner's Syndrome. These pictures show a Werner's syndrome patient at 15 and 48, demonstrating the rapid aging effects of the disease. Image from http://www.pathology.washington.edu/research/werner/ Permission Pending.
The Human RecQL Helicase Family
The gene encoding the WRN helicase, mutations of which have been implicated in Werner’s syndrome, is only one helicase in a family of related human helicase proteins. While many kinds of helicases exist in human cells, WRN specifically belongs to a family of helicases that are homologous to the E. coli RecQ helicase. All members of this family of helicases are all thought to be specifically involved in maintaining genomic stability and repairing DNA when problems or abnormalities arise during replication or recombination. (Chakraverty et. al., 1999). Members of this family share a highly conserved domain of 450 amino acids that provide both a helicase function in seven conserved domains and an ATPase function (Mohaghegh et al, 2001). The members of the human RecQL helicase family are listed below (Table 1).
Name |
Size |
Expression |
Other Features |
| RecQL |
Small |
Ubiquitous |
First discovered |
WRN (RecQL2) |
Large |
Tissue Specific |
Acidic N-terminal domain, exonuclease activity |
BLM (RecQL3) |
Large |
Tissue Specific |
Acidic N-terminal domain |
RecQL4 |
Large |
Tissue Specific |
Acidic C-terminal domain |
RecQL5 |
Small |
Ubiquitous |
Helicase domain comprises almost entire molecule |
Table
1. The members of the human RecQL helicase family. This
table lists the members of the helicase family that includes WRN and provides
a few measures of comparison between them (Kitao, 1998).
WRN Orthologs in Other Species
In addition to the other four related proteins found in humans, WRN is homologous to proteins in several other species and shares conserved helicase domains with them. Like the human family of helicases, several of these are thought to play a role in DNA maintenance and repair. The orthologs are listed below (Table 2).
Species |
Gene Name |
M. musculus |
Wrn |
E. coli |
RecQ (43% homology to WRN) |
S. cerevisiae |
SGS1 |
S. pombe |
RQH1 |
Table 2. Orthologs to the WRN gene in other species. This table lists some of the homologous proteins to WRN found in other species. These proteins share the helicase function of WRN. (Kitao, 1998) and (Sanger Institute: Ensembl Mouse Genome Server, 2003).
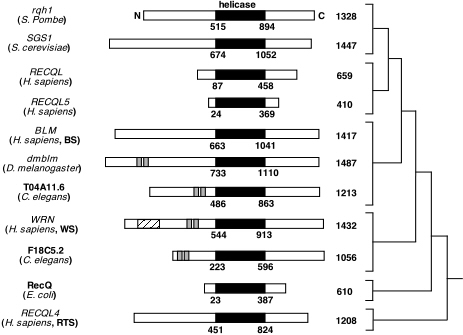
Figure 5. Summary of the RecQ family of helicases in humans and other species. This diagram shows many helicase genes that are homologous to WRN. The conserved helicase domain in the protein is shown shaded in black, and the degree of relatedness between the proteins is shown on the right. Image from: http://www.hms.harvard.edu/pathol/sinclair/Pages/recqfamily.html. Permission Pending.
Human Disorders Caused by Mutations of Other RecQL Family Members
In addition to sharing some aspects of structure and function with WRN, mutations of other members of the human RecQL helicase family may also cause diseases which, like Werner's syndrome, are related to a failure to repair DNA damage (Mohaghegh, 2001).
Bloom Syndrome
Bloom Syndrome is caused by mutations of the WRN homolog BLM. Like Werner’s
syndrome, cells of individuals suffering from this syndrome show an increased
accumulation of genomic instability and damage compared to normal cells (Kitao,
1998). Sufferers of this syndrome experience deficiency in growth both before
and after birth, sun-sensitivity, and, most importantly, an increased susceptibility
to cancer. (OMIM, 2002).
Rothmund-Thomson Syndrome
Mutations of another one of the proteins in the RecQL family of human helicases,
RecQL4, are responsible for Rothmund-Thomson Syndrome (RTS). This disorder is
primarily characterized by skin lesions and irritation resulting from photosensitivity
(Figure 6). Patients may also suffer from short stature. As with Werner's syndrome,
the problems seen in this disease likely result from a failure to repair DNA
damage in cells (Hsu, Sylvia, 2002).
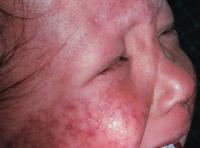
Figure 6. A Picture of an individual suffering from RTS. This image shows the skin lesions and damage caused by the photosensitivity of RTS, a disorder caused by a mutation in RecQL4. Image from http://www.emedicine.com/derm/topic379.htm Permission pending.
Links
OMIM: Werner Syndrome - The Online Mendelian Inheritance in Man site by NCBI about the syndrome caused by mutations of the WRN gene. Contains links to many articles related to the study of this protein.
Werner Syndrome: University of Washington - A website with information about Werner's syndrome and its clinical effects. Contains links to the WRN mutation database and the Werner's syndrome International Registry of information.
DNA Helicases Research - A website reviewing the future directions of research investigating human DNA helicase activity and particularly the function of WRN.
References
Alberts, et. al. 2002. Molecular Biology of the Cell- 4th ed. Garland Science, pp. 992-1010.
Chakraverty, Ronjon K. et. al. 1999. Defending genome integrity during DNA replication: a proposed role for RecQ family helicases [abstract]. Bioessays 21:286-294. <http://www3.interscience.wiley.com/cgi-bin/abstract/61001920/START> Accessed 2003 11 Mar.
Hsu, Sylvia, 2002. Rothmund-Thomson Syndrome. Available <http://www.emedicine.com/derm/topic379.htm> Accessed 2003 12 Mar.
Kitao, Saori et. al. 1998. Cloning of Two New Human Helicase Genes of the RecQ Family: Biological Significance of Multiple Species in Higher Eukaryotes. Genomics 54: 443-452.
Liu, et. al. 1999. The three-dimensional structure of the HRDC domain and implications for the Werner and Bloom syndrome proteins. Structure 7: 1557-66. Available <http://journals.bmn.com/journals/list/browse?uid=JSTR.st7c05&rendertype=text> Accessed 2003 12 Mar.
Mohaghegh, Payam et. al. 2001. The Bloom's and Werner's Syndrome Proteins are DNA Structure-specific Helicases. Nucleic Acids Research 29: 2843-2849.
Online Mendelian Inheritance in Man, OMIM (TM). Johns Hopkins University, Baltimore, MD. MIM Number: 210900: 2002 Nov. 1. World Wide Web URL <http://www.ncbi.nlm.nih.gov/omim/> (2003 Mar. 12)
Online Mendelian Inheritance in Man, OMIM (TM). Johns Hopkins University, Baltimore, MD. MIM Number: 604611: 2003 Jan. 7. World Wide Web URL <http://www.ncbi.nlm.nih.gov/omim/> (2003 Mar. 10)
Puranam, Kasturi L and Blackshear, Perry J. 1994. Cloning and Characterization of RecQL, a Potential Human Homologue of the Escherichia coli DNA Helicase RecQ. Journal of Biological Chemistry 269: 29838-45.
Sanger Institute, 2003. Ensembl Mouse Genome Server: Wrn. Available <http://www.ensembl.org/Mus_musculus/geneview?gene=ENSMUSG00000031583> Accessed 2003 11 Mar.
Sanger Institute, 2003. Ensembl Protein Report: WRN. Available <http://www.ensembl.org/Homo_sapiens/protview?peptide=ENSP00000298139&db=core> Accessed 2003 11 Mar.
Xue, Yu et. al. 2001. A Minimal Exonuclease Domain of WRN Forms a Hexamer on DNA and Possesses both 3'-5' Exonuclease and 5'-Protruding Strand Endonuclease Activities. Biochemistry 41: 2901-2912 Available <http://pubs.acs.org/cgi-bin/article.cgi/bichaw/2002/41/i09/pdf/bi0157161.pdf> Accessed 2003 13 Mar.
Questions or Comments? Please e-mail Rachel Patton McCord
Rachel Patton McCord's Molecular Homepage
Davidson College Molecular Biology