This webpage was created as an assignment for an undergraduate course at Davidson College.
Review of a Scientific Journal
The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs
Naokazu Inoue, Masahito Ikawa, Ayako Isotani & Masaru Okabe
Genome Information Research Center, Research Institute for Microbial Diseases, Faculty of Pharmaceutical Sciences, Osaka University, Yamadoaka 3-1, Suita, Osaka 565-0871, Japan
Nature, 10 March 2005. Vol. 434: 234-38.
Introduction
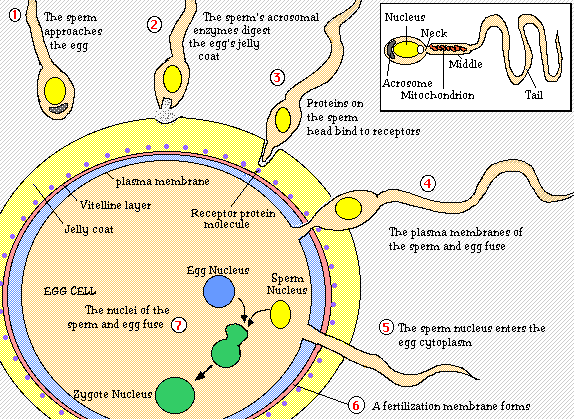
For fertilization to occur, a sperm must first make its way through two outer layers of the egg; the cumulus and the zona pellucida. To do this, the sperm head undergoes an acrosomal reaction in which enzymes located in the sperm’s acrosome digest the cumulus and the zona pellucida layers. The sperm then reaches the egg vitelline envelope where bindin protein on the sperm reacts with bindin receptors on the envelope. When these proteins recognize each other, the egg membrane swallows the sperm head. This allows the sperm nucleus to enter the egg’s cytoplasm and fuse with the egg nucleus (Purves et al, 2001).

Figure 1. Initial process of fertilization. This is the step by step process by which the sperm attaches to the egg and ultimately fuses with the egg's nucleus. A diagram of the sperm is shown at the top right-hand corner. Diagram taken from Sidwell Friends School.
This experiment sought to find the sperm protein involved in the fusion of egg and sperm during fertilization and classify it. From previous results, it is known that the monoclonal antibody, OBF13, prevents sperm from fusing with eggs. By performing two-dimensional gel electrophoresis to separate the many sperm proteins and immunoblotting the gel with OBF13, the researchers were able to identify and subsequently clone the sperm fusion protein involved in fertilization, Izumo.
Izumo was sequenced using liquid chromatography tandem mass spectrometry. Some of its peptide sequences were searched in the NCBI Database where it was determined that Izumo is part of the immunoglobulin superfamily. The DNA sequence registered in NCBI was confirmed as that for Izumo by amplifying Izumo RNA using polymerase chain reaction with reverse transcriptase (RT-PCR).
Summary of Results
Figure 1.
A. The mouse Izumo amino acid sequence is shown along with its human homologue. Besides noting the great amount of similarity between the human and mouse Izumo proteins, it is important to recognize the two domains colored in green found in both amino acid sequences indicating that Izumo is part of the immunoglobulin superfamily. The arrowheads above the cysteine groups in each immunoglobulin domain denote possible disulfide bridges. We can also see that the mouse Izumo, 397 amino acids in length, is longer than human Izumo which is 350 amino acids long.
B. The cartoon shows Izumo as an membrane protein containing an N-glycoside link and a disulfide bridge.
C. A Western blot using mouse Izumo protein and anti-mouse Izumo antibody is tested on tissues from different parts of the mouse’s body. The sperm sample in lane 11 is used as a positive control since it is certain that sperm contain Izumo. The blot expresses that Izumo is only found in the testes. This makes sense since the testes produce and house sperm. The length of the mouse Izumo is 56.4-kDa.
D. A Western blot of human Izumo is shown. We see that Izumo is also identified in human sperm. The size of human Izumo is 37.2-kDa, smaller than that of mice.
E. Mouse Izumo is immunostained with polyclonal anti-mouse Izumo antibody from sperm that exhibit green fluorescent protein in their acrosomes. Sperm that contain un-reacted acrosomes are not stained by the Izumo antibody (sperm color remains green), while sperm that contain reacted acrosomes are stained by the antibody (sperm color becomes red).
F. Human sperm are stained red with anti-human Izumo antibody and green in a separate staining with anti-CD46 antibody. CD46 is a protein located under the plasma membrane of sperm. It is used as both a positive and a negative control; you would expect to see it in acrosome reacted sperm, but not in acrosome un-reacted sperm. Since acrosome un-reacted sperm is also stained by anti-Izumo antibody, it is deduced that Izumo is located under the plasma membrane as well.
Figure 2.
A. Shown are the wild-type mouse Izumo allele, the targeting vector used to recombine with the wild-type allele, and the resulting mutant allele containing the “knocked-out” Izumo exons in that order from top to bottom. The wild-type allele contains both introns (horizontal lines) and exons (vertical boxes). The target vector contains a neomycin resistance gene as well as a gene for diphtheria toxin A. The resulting Izumo mutant allele’s exons fragment switched with the neomycin resistance gene. Both neomycin and diphtheria toxin genes act as controls to insure that a knocked-out Izumo allele was formed. Cells that have the diphtheria toxin gene in Izumo DNA will be killed from the produced toxin. Cells with alleles that have no neomycin/Izumo exon recombination will end up dying once placed in a medium that contains the drug neomycin.
B. The Southern blot reveals that there is a disruption in the Izumo allele when its exons have been replaced by the neomycin gene. While the wild-type Izumo allele is 15 kb long, the mutant is found to be 6.9 kb long. A 3’ external probe was used on all Izumo alleles.
C. A Northern blot is performed on all RNA located within mouse testes containing either wild-type Izumo (+/+), heterozygous Izumo (+/-), or homozygous Izumo (-/-) alleles. In the upper gel, the testes were screened for Izumo protein. All lanes, except the homozygous Izumo allele lane, contained Izumo protein within the testes RNA. The gel below that is a positive loading control containing the protein GADPH. We see that GADPH is expressed in all allele types.
D. Western blotting was performed on Izumo, ADAM2, CD147, and sp56 sperm proteins to assay whether or not disrupting the Izumo allele caused any disruptions among these proteins. ADAM2, CD147, and sp56 were not affected by disrupting the Izumo allele. The only lane that failed to show Izumo protein happened to be the homozygous Izumo allele lane, as expected. That means further experiments using disrupted Izumo will not affect other sperm proteins or results.
Figure 3.
A. The bar graph shows that male mice with (-/-) Izumo alleles are infertile and cannot produce any litters despite normal vigorous behavior. Males that are (+/-) for Izumo are also vigorous and can produce litters. This provides another example that Izumo is important in the fertilization process. Females are able to produce litters despite being Izumo (+/+) or Izumo (-/-). This is yet another example that Izumo protein only affects male mice.
B. The bar graph measured the frequency with which a pronucleus was formed after eggs were inseminated by either Izumo (+/-) or (-/-) sperm. Only Izumo (+/-) sperm were able to form a pronucleus with eggs. Izumo (-/-) sperm remained un-reactive.
C. In vitro fertilization shows that Izumo (+/-) sperm are able to fuse with the egg as expressed by the nearly non-existent amount of sperm surrounding the egg. Once the egg and sperm nuclei have fused, enzymes normally degrade receptors on the zona pellucida that allow sperm to attach to the egg plasma membrane. In vitro fertilization using Izumo (-/-) sperm shows many sperm collecting on the membrane of the egg. The impaired fertilization is most likely caused by the inability for the disrupted Izumo sperm to fuse with the egg nucleus.
D. Izumo (-/-) sperm are collected in the perivitelline space of the eggs in both panels. The lower panel is a clearer look at acrosome-reacted Izumo (-/-) stained with monoclonal antibody MN9 that have collected in the perivitelline space. Izumo-disrupted sperm make their way through the zona pellucida by under-going the acrosome reaction, but since they are uable to fuse with the egg, no signal comes from the fused zygote that stops this accumulation.
E. The bar graph represents the number of eggs fused with either Izumo (-/-) or Izumo (+/-) sperm after 2 hrs and 6 hrs. Eggs were fertilized when inseminated by the Izumo (+/-) sperm. No fertilization occurred when eggs were in the presence of Izumo (-/-) sperm.
F. Izumo (+/-) and (-/-) sperm were added to eggs that had their zona pellucida removed. Both types of sperm successfully bind to the egg's plasma membrane as seen in the first two panels. In the next two panels, Hoechst 33342 is used to stain and identify sperm fusing with eggs. The Izumo(+/-) sperm were found to fuse with eggs while the Izumo (-/-) did not.
Table 1.
Table 1 is used to further prove that disrupted Izumo sperm only affects the initial fusing process. By injecting the Izumo (+/-) and (-/-) sperm directly into the cytoplasm of eggs, the researchers were able to fertilize the eggs by bypassing the fusion process. Resulting embryos developed normally thereby supporting previous results that Izumo only affects egg-sperm fusion.
Figure 4.
A. In a xeno-fusion experiment, Izumo (+/-) and (-/-) mouse sperm is added to zona-free hamster eggs. Without the species-specific zona barrier surrounding the egg, Izumo (+/-) sperm is able to fuse with the hamster eggs as shown by staining with Hoechst 33342. When Izumo (-/-) sperm was added to the eggs, fusion did not take place. Again, this shows that a correctly functioning Izumo sperm is needed for fertlilization to take place.
B. Human sperm is added to zona-free hamster eggs in these panels. The two upper panels show that human sperm fuses with hamster eggs when treated with the control IgG antibody. In the bottom two panels, anti-human Izumo is added to the sperm. Since anti-hIzumo occupies the active site of Izumo and no fusion between eggs and sperm were found after staining with Hoechst 33342, this shows there is a direct and important relation between Izumo and fertilization. Only one protein was inhibited and as a result, the entire fertilization process did not occur.
Critique of Results
The researchers did a really clean job of presenting their data. Not only did the researchers deduce where Izumo resided, what it's structure and classification is, and determined a "knock-out" gene method of testing the Izumo protein, but they also carried out many experiments testing the function of Izumo. What was especially done well was the way each function test became more and more specific as the paper went on. Not only that, but each test was carefully controlled in its variables to make certain all the resulting conclusions about Izumo were backed by clear experimental evidence. It became harder and harder to doubt the results of the experiment the more one read each test.
I was not completely satisfied with some of the results, however. There were some things the researchers left out that kept this experiment from being perfect:
In Figure 1 C., despite seeing absolutely no Izumo protein in any of the tissues except the testes and sperm, there is no loading control present. We therefore cannot determine whether or not each lane was indeed loaded correctly or if there was some kind of contamination or mistake that occurred. If there was a loading contamination, then the researchers could be missing data on another possible tissue that produces Izumo.
In Figure 2 B., there is, again, no loading control to emphasize the difference between wild-type and mutant Izumo. The results of this blot are rendered useless without that important comparison.
In Figure 2 C., GADPH cannot be a loading control for Izumo because they are located on two different gels. The visible split between the Northern blot of Izumo and that of GADPH shows that the two results are on different gels and have simply been lined up.
In Figure 3 F., there is still a stained spot showing up in the Izumo (-/-) staining with Hoechst 33342. This could possibly mean that a fusion has gone on between an Izumo (-/-) sperm and an egg, but the researchers do not even mention it since it appears in the Izumo (+/-) staining as well. It is still strange that the researchers did not think about mentioning it within their text.
Future Work
Two future paths of research are mentioned at the end of the article. The first path can be research into the mechanism of sperm-egg fusion. Amazing as it may seem, how a sperm and egg fuse is still widely unknown. Before this paper, CD9, a main surface protein on eggs that regulates fusion, was the only fusion factor known. With Izumo being found as a fusion factor of sperm, the question arises whether these two fusion factors interact with one another and if so, how? Some methods to test this question come to mind. One could, for example, use a structural approach to see whether or not both proteins contain any complementary sequences that allow a direct binding between the two. Or one could use affinity chromatography to observe what parts of a sperm bind to the egg and vice versa. Discovery of such a mechanism is important because it will help provide insight to other cell-cell binding processes; including how trophoblasts interact with their environment to form the placenta, how stem cells are able to differentiate by using other cell structures as "templates", and how some types of diseases, like muscle rigidity, affect cells in the ways they do.
The other path that could be taken from this article is in the realm of contraceptive and fertility treatment. Contraceptive research, for example, could begin to address the fact that if either CD9 on eggs or Izumo in sperm is inhibited, then fertilization does not take place. Such treatments that target either of these proteins could possibly be cheaper and simpler to use than some contraceptive methods used today. However, ample research would need to be done to make sure that such contraceptive methods do not have significant drawbacks such as causing sterility in both males and females. Fertility treatments could likewise be proposed using the results of this paper. Knowing that Izumo and/or CD9 regulates fertilization, treatments including either protein could be made to increase the gametic prowess of either man or woman so that they may conceive.
References
Purves WK, Sadava D, Orians GH, Heller HC. Life: The Science of Biology. 6th Ed. Sinauer Associates, Inc., 2001
The Wellcome Trust. "The Human Genome Site." Accessed 27 April 2005. <http://www.wellcome.ac.uk/en/genome/technologies/hg17b013.html>.
Back to Davidson College Molecular Home
Any questions, comments, or suggestions?
E-mail pachampaloux@davidson.edu