This web page was produced as an assignment for an undergraduate course at Davidson College
MY FAVORITE PROTEIN
INSULIN
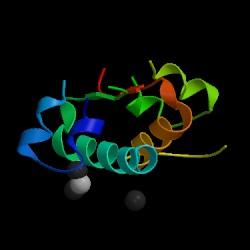
Figure 1. Structure of human insulin hormone by courtesy of the Protein Data Bank.
Sir Frederick Grant Banting and Charles Herbert Best earned a Nobel Prize for discovering the first horomone, insulin, in 1921 (Stryer, 1995). Insulin is a protein in the human body that plays a major role in decreasing the levels of glucose in the blood and regulating the metabolism of glucose, fats, and proteins (Lilly, 1996). There are different versions of the insulin protein, but this web page will focus on human insulin.
What does insulin look like?
Structure of Insulin
Insulin's empirical formula is: C254 H377 N65 O75 S6 and it has a molecular weight of 5734 (Wikipedia, 2005).
Insulin is built from 51 amino acids and is one of the smallest proteins in the body. It is structured with two polypeptide chains linked by two disulfide bonds, connecting the amino acid Cysteine to Cysteine. There is also a third disulfide bond that connects these same amino acids within Chain A. Chain A consists of 21 amino acids and chain B contains 30 amino acids. The one special thing about insulin is its change in structure to become useful in the human body. Insulin is originally produced as preproinsulin, which is transformed into a prohormone molecule by proteolytic action into proinsulin, and finally into the active polypeptide hormone, insulin (Bowen, 1999).
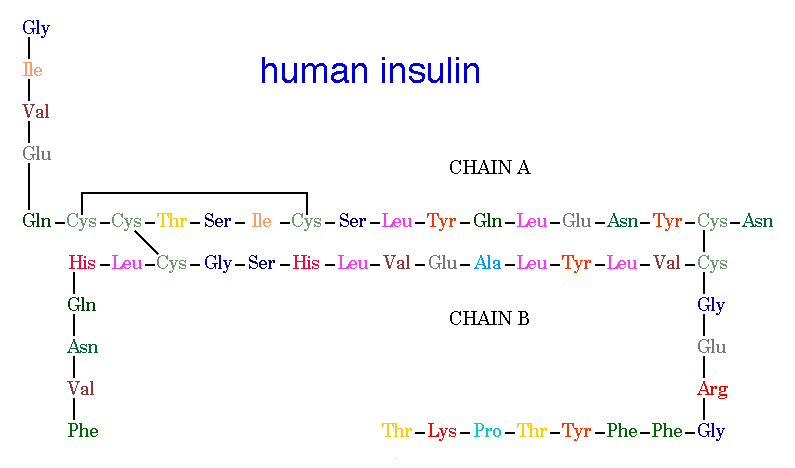
Figure 2. Human Insulin. The amino acid diagram of human insulin, showing the A and B chains and 3 disulfide bonds. Permission pending. http://www.chem.uwec.edu/Chem406/Webpages/Ying/overview.htm
The process: Preproinsulin --> Proinsulin --> Insulin
Once food enters the body, it immediately is detected and the insulin mRNA is translated as a single chain precursor called preproinsulin in the pancreas. By the removal of its signal peptide (see figure below) during insertion into the endoplasmic reticulum, proinsulin is generated. Preproinsulin is the primary translation product of the insulin gene, composed of 110 amino acids (Smith, et. al., 1997). It is relatively inactive and has to be processed into proinsulin in order to eventually make the insulin hormone. Within the endoplasmic reticulum, proinsulin is exposed to several specific endopeptidases to excise the C peptide chain (see figure below) of 31 amino acids from the single-stranded polypeptide to derive insulin. Now the mature form of insulin has been made into clusters of endocrine cells in inslets of Langorhans (Purves, et. al., 2001). The extra C peptide and signal peptide, which was clipped off, are packaged in the Golgi into secretory ganules to accumulate and be recycled in the cytoplasm. Once insulin is properly made and the beta cell is appropriately stimulated, insulin is secreated from the cell into the blood (Bowen, 1999).
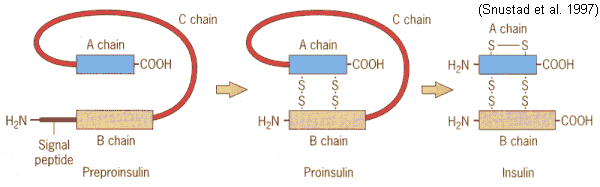
Figure 3. Converting preproinsulin to insulin. Preproinsulin is transcribed as a 110 amino acid chain and by the removal of the signal peptide, proinsulin is produced . Formation of disulfide bonds between the A- & B-chain components are made, and removal of the intervening C peptide chain produces biologically active Insulin of 51 amino acids. Permission pending. http://www.mun.ca/biology/scarr/Insulin_posttranslational_modification.htm
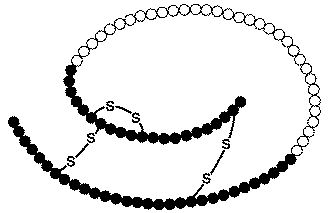
Figure 4. Proinsulin. Another look at an image of proinsulin, made of 86 amino acids with correct alignment of the three pairs of disulfide bonds. Permission pending. http://www.chem.uwec.edu/Chem406/Webpages/Ying/overview.htm
How does insulin work in the human body?
Insulin secretion
Insulin is synthesized in the beta cells of the pancreas through a glucose transporter by means of facilitated diffusion. The main function of the pancreas is to produce insulin, digestive enzymes, and other hormones (Norman, 2002). The secretion of insulin is controlled by the glucose concentrations in the blood stream. As the level of glucose rises in the blood, the insulin levels also increases. As carbohydrates or sugars are absorbed by the intestines after a meal, insulin is secreted by the pancreas in response to this increase in blood sugar. Due to the heightened levels of glucose, membrane depolarization of the beta cells occurs, causing extracellular calcium to rush into the cell. This in turn stimulates the export of secretory granules, which contain insulin, out of the cell (Bowen, 2004). Most cells through out the human body have insulin receptors to which insulin binds, and these cells activate other receptors designed to absorb the glucose from the blood stream. To elevate the glucose levels in the beta cells, calcium-independent pathways are triggered by these receptors. Insulin also works in the liver, muscle, and fat cells controlling glucose levels in the body (Purves, et. al., 2001). When glucose levels fall below normal range due to a lack of insulin, glycogen synthesis in the liver ceases and the enzymes responsible for the breakdown of glycogen become active (Bowen, 2004). And without insulin, many of our cells in the body would not be able to take up glucose and would have to resort to alternative fuels like fatty acids for energy (Norman, 2002).
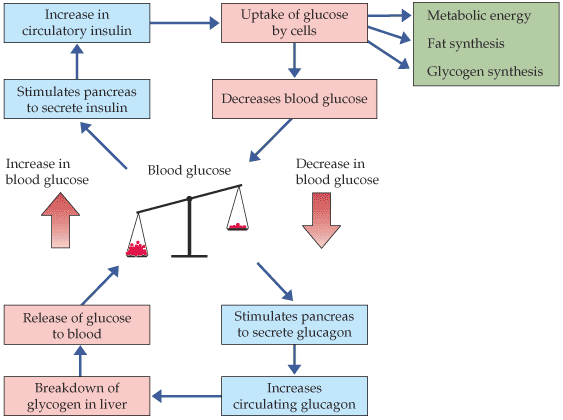
Figure 5. Diagram of the insulin and glucose regulation model. As blood sugar (glucose) rises, insulin is secreted in the pancreas, it is circulated through out the body, glucose is taken up by cells and blood sugar decreases. With a decrease in blood sugar, the pancrease secretes glucagon to breakdown glycogen in the liver and release glucose into the blood. This process maintains homeostasis in the body in reference to blood glucose levels (Roberts, 2003). Permission pending http://bcs.whfreeman.com.
Insulin receptors
Once insulin is secreted by the pancreas, it goes directly to the liver through the portal vein where it affects carbohydrate and lipid metabolism. This is where the action of the insulin receptors comes into place. The insulin receptors are tyrosine kinase, integral membrane proteins, which contain two alpha subunits and two beta subunits. The alpha subunits are entirely extracellular and hold the binding site for the insulin. The beta subunits are attached to each of the alpha subunits by a sulfur bonds and extend through the plasma membrane to anchor the protein in the cell wall. The two complexes are connected by a disulfide bond (Bowen, 2004).
When insulin binds to the alpha subunit, the beta subunits phosphorylate themselves. One of the substrates of the insulin receptor, IRS-1, is the most studied. This substrate serves as a docking center for activation of other enzymes to mediate insulin’s effects by a signal transduction process, creating a complex biological response.
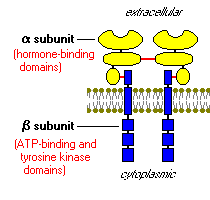
Figure 6. An insulin receptor. This insulin receptor has two alpha subunits (yellow) and two beta subunits (blue). The red lines are bonds, showing the sulfur bond between the alpha and beta subunits and a disulfide bond between the two complexes (Bowen, 2004). http://arbl.cvmbs.colostate.edu/hbooks/pathphys/endocrine/pancreas/insulin_phys.html
Carbohydrate and Lipid Metabolism effects
Glucose metabolism is regulated by insulin in different types of cells. During digestion in the small intestine, glucose is extracted from carbohydrates by hydrolysis into the blood stream. With the detection of increased levels of glucose in the blood, insulin is secreted by beta cells in the pancreas (Bowen, 2004). Once insulin binds the to the appropriate insulin receptors, cytoplasmic vesicles under the plasma membrane fuse with the plasma membrane and glucose transporters (Na+-independent and Na+-dependent) break through the plasma membrane. These glucose transporters are required for muscle, adipose, and some other tissues in the presence of insulin in order to transport glucose. Glucose also needs to be regulated and the phosphorylase A receptor measures the amounts of glucose present in the liver. With an influx of glucose, it binds to these receptors and alters the shape so it can be dephosphorylated and release the insulin to stimulate the formation of glycogen in the liver (Stryer, 1995). Glucose is changed into glycogen by the aid of insulin to ultimately maintain homeostasis in blood sugar (Roberts, 2003). The liver measures the amount of glucose in the blood stream and takes up or releases glucose as necessary (Stryer, 1995).
Whenever the liver builds up high levels of glycogen for the human body, glucose is absorbed from a food product and redirected into a different metabolic pathway. Fatty acids are made in this pathway and are transported out of the liver as lipoproteins. The lipoproteins produced are often used in other tissues, like adipose tissue, to make triglycerides. Insulin inhibits the breakdown of fat in adipose tissue by inhibiting the intracellular lipase that hydrolyzes triglycerides to release the fatty acids. Insulin is basically responsible for stimulating further accumulation of triglycerides in fat cells in adipose tissue (Bowen, 2004). Men from the Indian Institute of Chemical Biology have recently speculated that a new cell could be secreting insulin as well. Insulin is thought to only be secreted from beta cells in the pancreas, but now it is thought to exist in adipocytes. The structure-function relationship seem to be very similar and the amino acid sequences of AdpInsl and mammalian beta cell insulin can be related. As an insulin target cell, adipocytes can express the insulin gene and secrete the insulin protein to serve as a natural alternative to the beta cell insulin (Roy, 2003).
Other metabolic reactions insulin stimulates
- glucose transport
- amino acid transport
- glycogen synthase activity
- increases rate of general protein synthesis
- decreases lipolysis and protein degradation (Lilly, 1996).
How does insulin affect the human body?
Insulin regulates a variety of other cellular processes, such as protein and fat synthesis, RNA and DNA synthesis, cell growth, and differentiation. However, Insulin’s main concern is the regulation of glucose uptake in the “clinical manifestation of diabetes” (Pittman, et.al., 2004). High levels of glucose will cause water to move from cells in the body to the blood by osmosis and the kidneys will increase in urine output to excrete excess fluid volume from the blood. This is why diabetes is concerned with issues of an overproduction of "sweet" urine (Purves, et. al., 2001). Also, with insufficient amounts of insulin or a mutation within the insulin protein, different diseases can develop like diabetes. There are two main types of diabetes mellitus, idiopathic and secondary. Idiopathic diabetes is divided into two subgroups, Type I and II, and secondary diabetes has many causes that can lead to a host of other diseases. We are most accustomed to idiopathic diabetes, Type I and II.
Idiopathic Diabetes, Type I
Insulin dependent diabetes (IDDM) usually manifests in childhood (juvenile-onset diabetes) and is a result of an autoimmune destruction of the beta calls in the pancreas (King, 2004). This type of diabetes affects about 2 million people in the United States. Within these patients, there is an insufficiency of insulin and a supplement of insulin is necessary by injection. It is known to be genetic, but the actual reason to the destruction of the beta cells is unknown.
Idiopathic Diabetes, Type II
Non-insulin dependent diabetes (NIDDM) is characterized by persistent hyperglycermia. It usually manifests in people over the age of 40 (adult-onset diabetes). It can result from a genetic defect and can cause insulin resistance or insulin deficiency, one associated with obesity and one not associated with obesity (King, 2004). Type II diabetes affects about 85% of the total diabetic population. For these patients obesity seems to be a common issue because of the increase in fat from adipose tissue. Therefore, the main form of treatment is a low carbohydrate diet (little sugar) and an exercise program (Vander, 1998).
Secondary Diabetes
This type of diabetes can result in many causes including:
- Maturity onset diabetes of the young (MODY); has an onset prior to age 25 with shown impairment of beta cell function and late beta cell failure; there are six types
- Pancreatic disease
- Endocrine disease
- Drug-induced diabetes
- Mutations in the insulin gene
- Mutations in insulin receptor gene which can lead to Leprachaunism, Rabson-Mendenhall syndrome, and Type A insulin resistance
- Gestational diabetes, shown during pregnancy and following childbirth
- Other disease involving impaired glucose tolerance (King, 2004).
References
Bowen, R. 1999 June 15. Insulin Synthesis and Secretion. <http://arbl.cvmbs.colostate.edu/hbooks/pathphys/endocrine/pancreas/insulin.html>. Accessed 2005 Feb 12.
Bowen, R. 2004 Oct 14. <http://arbl.cvmbs.colostate.edu/hbooks/pathphys/endocrine/pancreas/insulin_phys.html>. Accessed 2005 Feb 12.
King, Michael. 2001 June 15. Insulin Function and Diabetes. <http://web.indstate.edu/theme/mwking/diabetes.html>. Accessed 2005 Feb 10.
Lilly, Eli. 1996 Dec 13. Insulin Biosynthesis and its Hormonal Functions. Homepage.<http://www.chem.uwec.edu/Chem406/Webpages/ Ting/overview. htm>. Accessed 2005 Feb 10.
Norman, James. 2002. What is insulin? Endocrine Web’s Diabetes Center. <http://www.endocrineweb.com/diabetes/2insulin.html>. Accessed 2005 Feb 10.
Pittman, I.. L. Philipson, D. Steiner. 2004 Nov. Insulin Biosynthesis, Secretion, Structure, and Structure-Activity Relationships.<http://www.endotext.org/diabetes/diabetes3_new/ diabetes3.htm>. Accessed 2005 Feb 10.
Roberts, R.G., C.P.F. Redfern, T.H.J. Goodship. 2003 July 10. Effect of insulin upon protein degradation in cultured human myocytes. European Journal of Clinical Investigation. 33, 861-867.
Roy, S.S., et. al. 2003. A New Cell Secreting Insulin. Endocrinology. 144(4), 1585-1593.
Smith, A.D., et. al., ed. 1997. Insulin. Oxford Dictionary of Biochemistry and Molecular Biology. Oxford: Oxford University Press, 334.
Stryer, Lupert. Biochemsitry. New York: W.H. Freeman and Co., 1995.
Vander, Arthur, et. al. Human Physiology: the Mechanisms of Body Movement. Boston: McGraw-Hill, 1998.
Wikipedia: The Free Encyclopedia. 2005 Feb 7. <http://en.wikipedia.org/wiki/Insulin>. Accessed 2005 Feb 14.
Davidson College Biology Home Page
© Copyright 2005 Department of Biology, Davidson College, Davidson, NC 28036
If you have any questions, comments, or suggestions concerning this page, please contact kedresser@davidson.edu