"This web page was produced as an assignment for an undergraduate course at Davidson College."
Hemoglobin
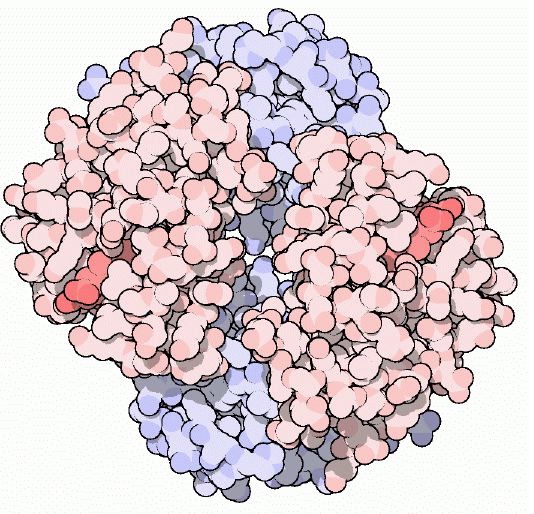
Figure 1: Cartoon drawing of the hemoglobin molecule. One alpha-beta dimer is colored red and the other is colored blue.The darker red represents the heme molecules. Image courtesy of Protein Data Bank
Basic Facts About Hemoglobin
Hemoglobin is found in the red blood cells of the body. Each red blood cell (RBC) contains approximately 280 million hemoglobin molecules (Sears, 1999). The main function of hemoglobin is to transport oxygen from the lungs to the tissues and then transport CO2 back from the tissues to the lungs. One hemoglobin molecule has the ability to transport up to 4 oxygen molecules. There are two forms of hemoglobin: oxyhemoglobin, which is saturated with oxygen molecules and deoxyhemoglobin, which is desaturated with oxygen molecules (Sears, 1999). Oxyhemoglobin has a higher affinity for oxygen than deoxyhemoglobin, and deoxyhemoglobin has a higher affinity for CO2 than oxyhemoglobin. Therefore, oxygen binds to oxyhemoglobin in the lungs and is then transported through the blood stream until it reaches the tissues. There, the oxygen is released to myoglobin, which then transports it to the mitochondria where it is used for aerobic respiration. In exchange, the deoxyhemoglobin picks up 2 protons and 2 molecules of CO2 and returns to the lungs, where the CO2 is released through exhalation (Perutz, 1978).
Structure of Hemoglobin
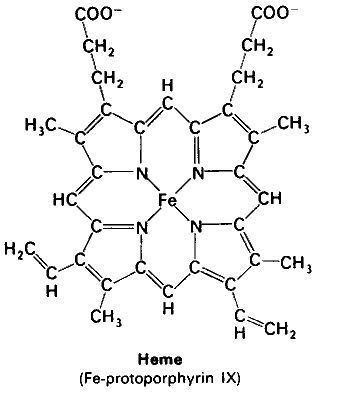
Hemoglobin is a tetramer composed of 4 globin molecules; 2 alpha globins and 2 beta globins. The alpha globin chain is composed of 141 amino acids and the beta globin chain is composed of 146 amino acids (Perutz, 1978). Both alpha and beta globin proteins share similar secondary and tertiary structures, each with 8 helical segments (labeled helix A-G) (Keates, 2004). Each globin chain also contains one heme molecule. The heme molecule is composed of a porphyrin ring, which contains 4 pyrrole molecules cyclically linked together, and an iron ion ligand bound in the center. The heme molecule is located between helix E and helix F of the globin protein. The alpha and beta subunits of the globin chains exist in two dimers which are bonded together strongly (Keates, 2004).
Figure 2: 3-D Ribbon Structure of the hemoglobin molecule. Each of the 4 globin chains is represented in a different color. The heme molecule is shown in red. http://www.psc.edu/science/Ho/Ho.html#hemoglobin (permission pending). |
|
Oxygen binds to the iron ion tightly, and if two heme molecules come together in the presence of oxygen the iron atoms will oxidize and irreversibly bind to the oxygen. This irreversible binding would not be of use in the hemoglobin molecule because oxygen needs to be released in the tissues. The globin chain prevents this irreversible binding by folding the protein around the heme molecule, creating a pocket to isolate the heme molecule from other heme molecules (Perutz, 1978). Therefore, the globin molecules allow the iron atom to form loose bonds with the oxygen, and therefore, the ability to bind to oxygen and then release it into the tissues without becoming permanently oxidized in the process.
Function of Hemoglobin
The ability of hemoglobin to take up oxygen molecules in the lungs and then release them in the tissues is regulated by several factors both within the hemoglobin molecule itself and through external chemical factors. One of the biggest regulators of the oxygen affinity of the hemoglobin is the presence of oxygen itself. In the lungs where the oxygen levels are high, the hemoglobin has a higher affinity for oxygen and this affinity increases disproportionately with the number of molecules it already has bound to it (Sears, 1999). In other words, after the oxyhemoglobin binds one molecule of oxygen its affinity for oxygen increases until the hemoglobin is fully saturated. In the same way, the deoxyhemoglobin has a lower affinity for oxygen and this affinity decreases disproportionately with the number of molecules it already has bound (Sears, 1999). Thus, the loss of one oxygen molecule from the deoxyhemoglobin lowers the affinity for the remaining oxygen. This regulation is known as cooperativity and is essential to the functioning of the hemoglobin because it allows the oxyhemoglobin to carry the maximum amount of oxygen to the tissues and then allows the deoxyhemoglobin to release the maximum amount of oxygen into the tissues (Sears, 1999). Cooperativity is a function of the hemoglobin's unique structural characteristics, and it was found that the cooperative effects of the hemoglobin totally disappear if the hemoglobin is split in half (Perutz, 1978). Essentially, hemoglobin is an allosetric protein that has more than one shape and can undergo conformational changes in its structure based on environment conditions (Sears, 1999). There are two alternative structures of hemoglobin; the relaxed structure (R) which has a greater oxygen affinity, and the tense structure (T) which has lower affinity for oxygen (Perutz, 1978). The change between the T and R structures is the result of a rotation of 15 degrees between the two alpha-beta dimers (Keates, 2004). To view an animation of this rotation click here. This rotation changes the bonds between the side chains of the alpha-beta dimers in the F helix and therefore causes the heme molecule to change positions. In the T structure, the iron ion is pulled out of the plane of the porphyrin ring and becomes less accessible for oxygen to bind to it, thus reducing its affinity to oxygen. In the R structure the iron atom is in the plane of the porphyrin ring and is accessible to bind oxygen, thus increasing its oxygen affinity (Keates, 2004). The transformation from the T to R structure occurs when oxygen binds to the T structure under the high oxygen pressure environment in the lungs, which causes the rotation of the two dimers and shifts the remain iron atoms so that they become more accessible to oxygen. Likewise, the transformation from the R to T structure occurs when oxygen is released under the low oxygen pressure environment of the tissues which causes the dimers to rotate back and shifts the iron atoms so that they become less accessible to oxygen (Keates, 2004). Thus, the cooperativity of the hemoglobin molecule can be explained by its unique structure which allows it to shift between the T and R structures in the presence or absence of oxygen. To see an animation of the whole hemoglobin molecule in action click here.
The oxygen affinity of hemoglobin can also be regulated by external chemical factors including pH, carbon dioxide, and DPG (2,3-diphosphoglycerate). In general any chemical agents that strengthen the bonds between the alpha subunits and prevent the rotation to the R structure decrease the oxygen affinity of the hemoglobin (Perutz, 1978). When CO2 is released into the blood from the tissues it acidifies the blood by increasing the concentration of hydrogen ions. This lowering in pH causes the oxygen affinity of the hemoglobin to decrease, which is known as the Bohr effect. The molecular basis behind the Bohr effect is that the T structure of hemoglobin binds hydrogen more readily than the R structure, so under a condition of low pH (high hydrogen ion concentration) the T structure, which has a decreased oxygen affinity, dominates (Keates, 2004). CO2 has a similar effect on the hemoglobin, but instead of binding to the heme molecule like oxygen, CO2 binds to the N-terminus of the alpha globin molecule (Keates, 2004). The CO2 binds better to the globin in the T structure, so the release of oxygen in the tissues by the T structure of hemoglobin facilitates the uptake of CO2. Then in the lungs, the uptake of oxygen causes the hemoglobin to change to the R structure, which causes the release of the CO2 into the lungs, because CO2 does not bind as well to the R structure (Keates, 2004). Finally, DPG is an allosteric effector that changes the oxygen affinity of hemoglobin by binding to the hemoglobin itself. DPG can bind to the T structure of hemoglobin, because of the change in structural conformation which allows it to fit, but can't bind to hemoglobin in the R structure. Therefore, the presence of DPG lowers the oxygen affinity by keeping the hemoglobin in the T structure longer (Keates, 2004).
Adaptation of Bird Hemoglobins to High Altitudes
There are several species of animals that are adapted to live at high altitudes. One of the main problems of living at such altitudes is the decrease in oxygen pressure, and as a result many species have evolved hemoglobins that are specially adapted to increase oxygen affinity (Liang et al., 2001). The bar-headed goose is one such species that lives around Tibetan lakes at altitudes from 4000-6000 meters and has even been observed flying over the summit of Everest (Jessen et al. 1991). The hemoglobins of the bar-headed goose have been sequenced and compared to close relatives that do not live at high elevations and it was found that there were 4 main mutations between the hemoglobin molecules. The main mutation resulted in a proline being replace by an alanine, which leaves a two-carbon gap between the alpha-beta dimer bonds. This gap relaxes the T structure and allows the iron ion to be more in the plane of the porphyrin ring, which raises its oxygen affinity of the deoxyhemoglobin and therefore allows it to bind oxygen more readily under lower pressures (Jessen et al. 1991). It was also found that other high altitude birds have similar mutations but on different globin chains, which suggests that the evolution of hemoglobin with increased oxygen affinity may have occurred more than once (Jessen et al. 1991). Thus, it is apparent that the structure of hemoglobin is essential to its function as it determines its oxygen affinity and binding capabilities.
References
Ho, Chien and Marcela Madrid. New Blood: Substitutions in Mutants of Hemoglobin. http://www.psc.edu/science/Ho/Ho.html#hemoglobin. February 2005.
Jessen, Timm H et al. 1991. Adaptation of bird hemoglobins to high altitudes: Demonstration of molecular mechanism by protein engineering. Evolution 88: 6519-6522.
Keates, Dr. R.A.B. 2004. Lecture 2, 3, 4. Chem 3560: Structure and Function in Biochemistry. Department of Chemistry and Biochemistry: University of Guelph. http://www.chembio.uoguelph.ca/educmat/chm356/index.htm. February 2005.
Liang, Yuhe et al. 2001. The Crystal Structure of Bar-headed Goose Hemoglobin in Deoxy Form: The Alloseteric Mechanism of a Hemoglobin Species with High Oxygen Affinity. Journal of Molecular Biology 313: 123-137.
Perutz, M.F. 1978. Hemoglobin Structure and Respiratory Transport. Scientific American 239 (6).
Protein Data Bank. http://www.rcsb.org/pdb/molecules/pdb41_1.html. February 2005.
Sears, Duane W. 1999. Overview of Hemoglobin's Structure/Function Relationships. http://tutor.lscf.ucsb.edu/instdev/sears/biochemistry/tw-hbn/hba-overview.htm. February 2005.
© Copyright 2005 Department of Biology, Davidson College, Davidson, NC 28036
Send comments, questions, and suggestions to: krheiner@davidson.edu