"This web page was produced as an assignment for an undergraduate course at Davidson College."
Hemoglobin Orthologs
Orthologs are sequences of genes that evolved from a common ancestor and can be traced evolutionarily through different species. By comparing the ortholog sequences of a specific gene between many species, the amino acid sequences which are conserved can be determined. These highly conserved sequences are important, because they provide information on which amino acids are essential to the protein structure and function.
Evolution of Hemoglobin
Hemoglobin is derived from the myoglobin protein, and ancestral species just had myoglobin for oxygen transport. 500 million years ago the myoglobin gene duplicated and part of the gene became hemoglobin. Lampreys are the most ancestral animal to have hemoglobin, and the ancestral version was composed of dimers instead of tetramers and was only weakly cooperative. 100 million years later, the hemoglobin gene duplicated again forming alpha and beta subunits. This form of derived hemoglobin is found in bony fish, reptiles, and mammals, which all have both alpha and beta subunits to form a tetramer (Mathews et al., 2000).
Conserved Sequences
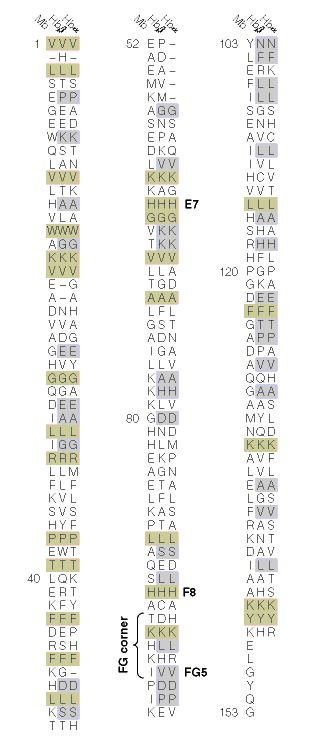
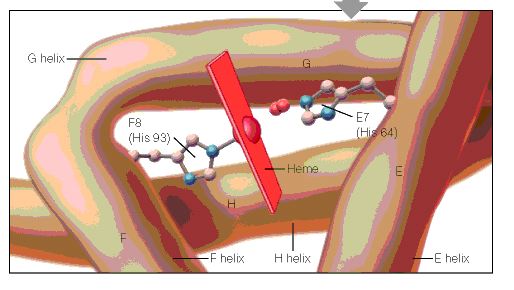
When the amino acid sequences of myoglobin, the hemoglobin alpha subunit, and the hemoglobin beta subunit are compared, there are several amino acids that remain conserved between all three globins (Mathews et al., 2000). These amino acid sequences are considered truly essential, because they have remained unchanged throughout evolution, and therefore are fundamental to the function of the protein. These essential amino acids can be seen in Figure 1, which compares myoglobin, and the alpha and beta subunits of hemoglobin. The histidines in helix F8 and helix E7 are highly conserved. These histidines are located proximally and distally to the heme molecule and keep the heme molecule in place within the hemoglobin protein as seen in Figure 2 (Mathews et al., 2000). This shows that the position of the heme molecule within the globin protein is essential to its function. Likewise, the amino acids in the FG region are also highly conserved. This region of the protein is essential to the conformational change between the T to R states (Mathews et al., 2000). Additionally, the amino acids at the alpha-beta subunit interfaces are highly conserved, because they also affect the conformational change between the subunits, which regulates oxygen affinity and cooperativity. In general, the most highly conserved sequences are located within the interior of the hemoglobin protein where the subunits contact each other (Gribaldo et al., 2003).
|
Figure 1: The amino acid sequences of myoglobin, alpha subunit of hemoglobin, and beta subunit of hemoglobin. The amino acid sequences highlighted in tan are conserved between all three globins and the amino acid sequences highlighted in gray are conserved between alpha and beta hemoglobin. http://www.aw-bc.com/mathews/ch07/fi7p11.htm(permission pending). |
Figure 2: A cartoon drawing of the structure of hemoglobin around heme molecule. The histadines in helix F8 and E7 interact directly with the heme molecule. http://www.aw-bc.com/mathews/ch07/fi7p5.htm (permission pending). |
Alpha Subunit of Hemoglobin
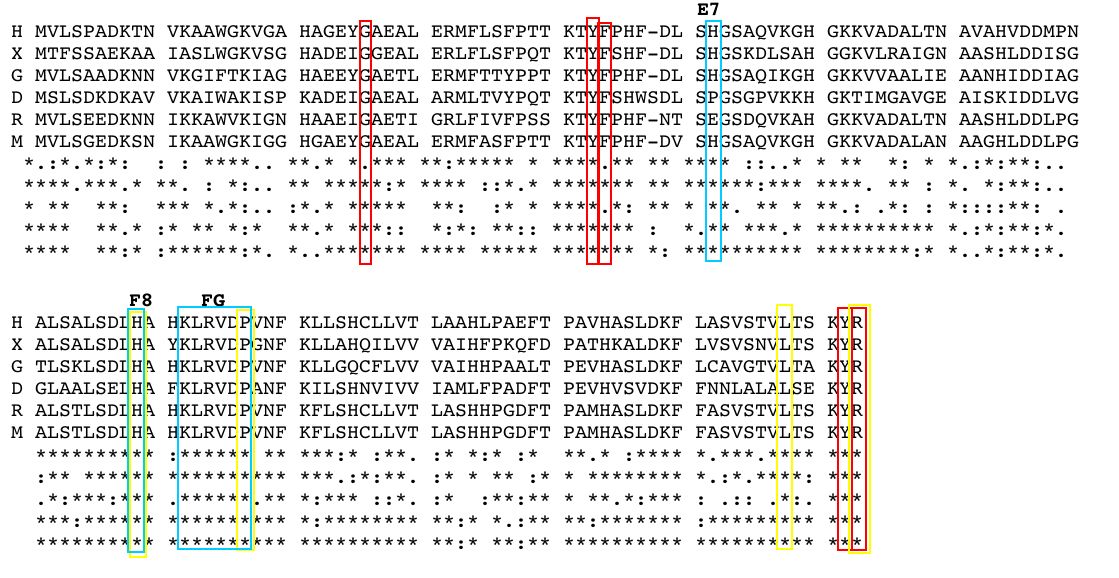
The alpha subunit of hemoglobin has several amino acid sequences that are conserved across many species and are essential to its function. The alpha subunit of hemoglobin is encoded by the 2 genes HBA1 and HBA2 both located on chromosome 16 (GeneCard, 2005). Click here to see the gene card for HBA1. To determine which amino acid sequences are conserved, I compared the orthologs of HBA1 in Homo sapiens (humans) to 5 additional species including, Xenopus tropicalis (African clawed frog), Danio rerio (Zebra fish), Gallus gallus (Red jungle fowl), Mus musculus (mouse), and Rattus norvegicus (rat) using the Ensembl program. Figure 3 shows the 6 orthologs aligned and the important conserved regions highlighted. The stars indicate amino acids that are conserved between all of the species. As a general observation, the mouse ortholog of HBA is the most similar to human HBA, because it is the most evolutionarily related. The amino acid sequences that are conserved in all globin proteins (highlighted in blue) can be seen in Figure 3. There are also several conserved amino acids that are specifically important to HBA structure (highlighted in red) including: the phenylalanine (F) at position 44, which is in direct contact with the heme group; tyrosine (Y) at position 142, which stabilizes the hemoglobin molecule by forming hydrogen bonds between two of the helices; and glycine (G) at position 26, which is small and therefore allows two of the helices to approach each other, which is important to the structure of hemoglobin (Natzke, 1998). Additionally, there are several proteins found in the alpha subunit that are involved in the movement of the alpha and beta subunits (also highlighted in red) including: the tyrosine (Y) at position 43, which interacts with the beta subunit during the R state, and the arginine (N) at position 143, which interacts with the beta subunit during the T state (Gribaldo et al., 2003).
|
Figure 3: Orthologs of the hemoglobin alpha subunit (HBA) from 6 species: H (Homo sapiens), X (Xenopus tropicalis), G (Gallus gallus) D (Danio rerio), R (Rattus norvegicus), and M (Mus musculus). The stars indicate conserved sequences between all species. The blue highlighted regions indicate amino acid sequences that are conserved between all globin proteins. The red highlighted regions indicate amino acid sequences that are conserved in HBA. The yellow highlighted regions indicate the position of key mutation is conserved portions of HBA. Amino acid sequences obtained from and aligned with Ensembl. |
Mutations
Looking at the effects mutated portions of a gene is also a good way to determine the function of highly conserved sequences. In hemoglobin, deleterious mutations are most common in the heme pockets of the protein and in the alpha and beta subunit interfaces (Mathews et al., 2000). There are several key mutations in highly conserved portions of HBA (highlighted in yellow) including: the substitution of histidine (H) at position 88 to tyrosine (Y), which disrupts the heme molecule leading to decreased oxygen affinity; the substitution of arginine (N) at position 143 to histidine (H), which eliminates a bond in the T state and therefore favors the R state, resulting in increased oxygen affinity; the substitution of proline (P) at position 97 to arginine (N), which alters the alpha-beta contact region and results in the disassociation of the hemoglobin complex; and the substitution of leucine (L) at position 138 for proline (P), which interrupts the helix formation and also results in the disassociation of the hemoglobin complex (Mathews et al., 2000).
Bar-headed Goose Hemoglobin
As mentioned on the previous page, the bar-headed goose has hemoglobin that is specifically adapted to high altitudes. The bar-headed goose hemoglobin has an increased oxygen affinity which allows it to live in low oxygen pressure environments (Liang et al., 2001). This increased oxygen affinity is the result of a mutation at position 121 in the alpha subunit, which is highly conserved in other species, from proline to alanine, as seen in Figure 4 (Liang et al., 2001). This substitution leaves a two-carbon gap between the alpha-beta dimer, which relaxes the T structure and allows it to bind oxygen more readily under lower pressures (Jessen et al. 1991). Thus, comparing orthologs can also be used to explain differences in the oxygen binding capabilities of hemoglobin in different species.
Figure 4: Orthologs of hemoglobin alpha subunit (HBA) from H (Homo sapiens) and B (Bar-headed goose). The red highlighted region indicates the position of the mutation from proline (P) to alanine (A). Amino acid sequences obtained from and aligned with Ensembl. |
References
Ensembl. Ensembl Genome Browser. http://www.ensembl.org/. Accessed March 2005.
GeneCard. 2005. GeneCard for HBA1. http://genome-www.stanford.edu/cgi-bin/genecards/carddisp?HBA1&search=HBA&suff=txt. Accessed March 2005.
Gribaldo, Simonetta, Didier Casane, Philippe Lopez and Herve Philippe. 2003. Functional Divergence Prediction from Evolutionary Analysis: A Case Study of Vertebrate Hemoglobin. Molecular Biology and Evolution 20 (11): 1754-1759.
Jessen, Timm H et al. 1991. Adaptation of bird hemoglobins to high altitudes: Demonstration of molecular mechanism by protein engineering. Evolution 88: 6519-6522.
Liang, Yuhe et al. 2001. The Crystal Structure of Bar-headed Goose Hemoglobin in Deoxy Form: The Alloseteric Mechanism of a Hemoglobin Species with High Oxygen Affinity. Journal of Molecular Biology 313: 123-137.
Mathews, Christopher, Kensal Van Holde and Kevin Ahern. 2000. Biochemistry 3 rd edition. http://www.aw-bc.com/mathews/ch07/c07emhp.htm . Accessed March 2005.
Natzke, Lisa. 1998. Hemoglobin. http://biology.kenyon.edu/BMB/Chime/Lisa/FRAMES/hemetext.htm. Accessed March 2005.
© Copyright 2005 Department of Biology, Davidson College, Davidson, NC 28036
Send comments, questions, and suggestions to: krheiner@davidson.edu