*This website was produced as an assignment for an undergratuate course at Davidson College.*
T-Cell Surface Glycoprotein CD4 Structure and Function
Through analyzing the paper "Kinetic and structural analysis of mutant CD4 receptors that are defective in HIV gp120 binding" (Click Here To View Article) which explores the function of the mutated CD4 protein in regards to the binding of HIV gp120 to the CD4 protein sites in T-Cells, I will relate the protein's structure to its function in the cell. Past studies have shown that the study of CD4's structure, as it relates to its function, is important due to the proteins role in the contraction of the Human Immunodeficiency Virus or HIV. The structure of the CD4 can actually allow, or prohibit, the HIV virus from binding and infecting the T-Cells. This ultimately causes the problems that are associated with the virus making this protein an area of high interest.

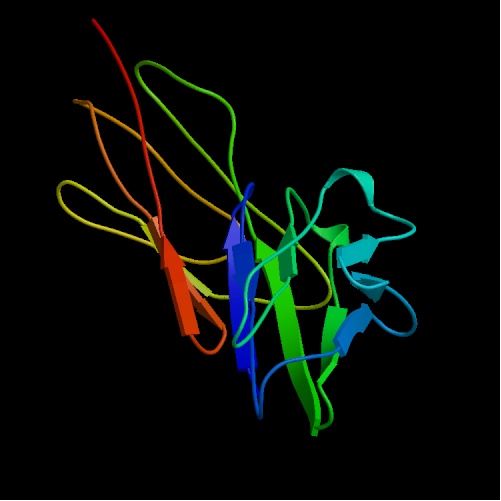
Figure 1: Structure of CD4. V-Domains and C-Domains are visible as V type red/orange C type green/blue. Picture courtesy PDB
Structure:
Before we can understand the function of the CD4 protein we must first look at the protein's structure. CD4 consists of 428 amino acids total that are distributed into segments as follows: 372 amino acid extracellular segment, 23 amino acid transmembrane segment, and a 33 amino acid cytoplasmic segment (Wu, 1996). The extracellular region of the CD4 protein is a single chain molecule that is made up of four tandemly arranged immunoglobulin-like domains (Fig. 1). These domains are catagorized by their relation to the membrane in so that there is a two domain segment called the membrane distal fragment (Which consists of Domain 1, or D1, and Domain 2 (D2)), as well as a two domain segment called the membrane proximal fragment (D3 and D4) (Wu, 1996). The domain that is of intrest, when it comes to HIV and the gp120 protein, is domain D1. This domain is homologous to the variable domain of antibodies that exhibit the structure of two Beta sheets four or five strands long (Wu, 1996). The stucture of D1 (as well as D3) is consistant with that of V-Domain (Figure 2, part B), while D2 and D4 are catagorized as C-Domains. The V, or variable domain, is often accompanied by a joining region (Wu, 1996). The two combined regions are classified as the V-J region and it is at this region (known as D1) that CD4 binds to MHC class II molecules which I will discuss further in the function segment of this page.
Figure B
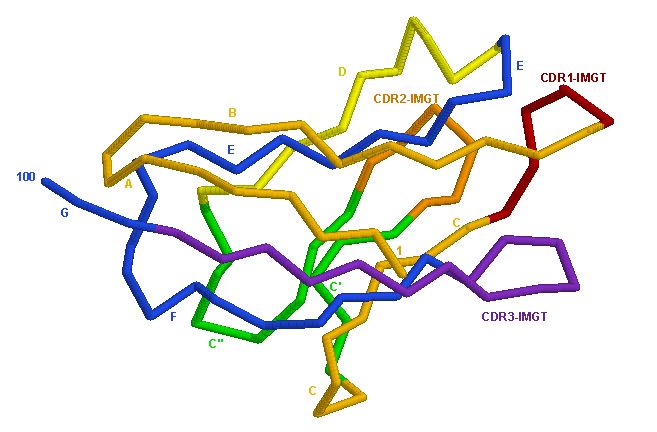
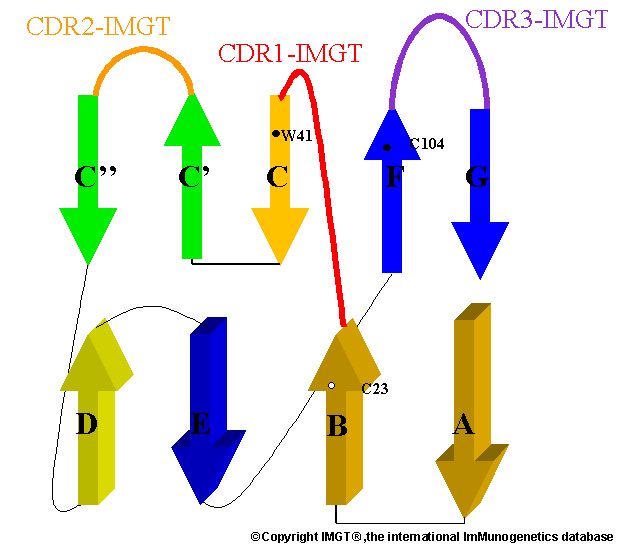
Figure A
Figure 2. (A) Structure of CD4's D1 domain. Structure relates to the V-Domain. (B) Simplified illustration of a V-Domain. The binding site of CD4 to gp120 occurs at the V-J site (Figure A). Picture courtesy Marie-Paule Lefranc (permission pending).
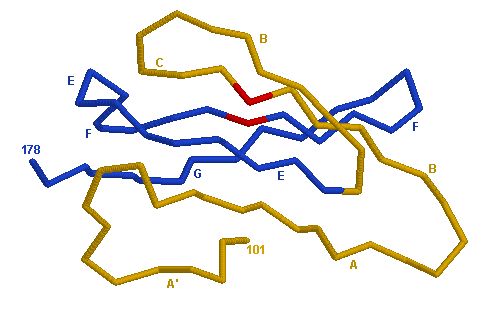
Figure 3: Illustration of Domain 2 (D2). The difference between a C-Domain and a V-J Domain is obvious in the structural differences between Figure 1 A and Figure 3. Picture courtesy Marie-Paule Lefranc (permission pending).
Function:
Now that we have determined the structure of the T-cell surface glycoprotein CD4 we can now begin to undertand the function of the protein. As mentioned above, the CD4 has tandem extracellular domains (D1, D2, D3, and D4) (Fig 1). In a normal, virus free organism, these termini are used by CD4 for anitgen recognition when associated with extracellular major histocompatibility complex class II (MHC class II) (Wu,1996). In past studies, it has been shown that CD4 is the primary binding site for the antigen T4/leu3 (Wu, 1996). Although this reaction occurs extracellularly, CD4 can also undergo intracellular reactions with the src-like lymphocyte tyrosine kinase (Lck). When viewing the structure of the T4/leu3, we can see that the antigen consists of a V-J Domain region that is homologous to the MHC class II complex (Wu, 1996). What this tells us is that the structure of the antigen is very important for its recognition by CD4. Now that we have established that a V-J style domain is easily recognized by CD4's D1 V-J Domain, we can take a look at how CD4 binds to gp120. According to the paper, the main site for the bonding occurs at the D1 domain terminus that has a V-J Domain structure. Although the structure of gp120 is variable, its structure is recognized by the CD4 protein and an electrostatic bond occurs between the two. It is from this bond that the HIV virus inserts its DNA into the T-Cell and causes the severe problems associated with the disease. According to the paper, the most important aspect of this bond is the structure of the protein. In the paper, the scientists manipulated the shape of the D1 domain of CD4 in various ways to see if the resulting structure would have an effect on the rate of binding to gp120. As predicted, when the shape of the domain was changed, the rate of binding changed 50 fold in some instances and 200 fold in others (Wu, 1996). Although the exact interaction between the two proteins is still unknown, it has been established that the structure of the CD4 protein is very much a vital aspect in its ability to bind with gp120 (Wu, 1996).
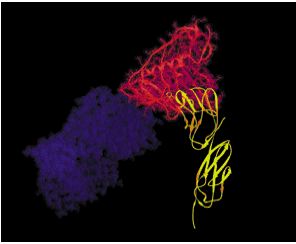
Figure 3: An illustration of the binding of gp120 sites of HIV to the CD4 D1 domain. Picture courtesy Howard Hughes Medical Institute (Permission Pending).
Mutations: Studies have confirmed that mutation in the CD4 protein as well as mutations in which the CD4 protein is not produced, have caused certain individuals to not acquire AIDS although they have been repeatedly exposed to the HIV virus. These mutations in the structure of the protein have protected certain individuals from the binding of gp120 to the CD4 proteins in the T-Cells.
References:
Lefrance, M. 3D Representation: Human CD4 Domain.http://imgt.cines.fr/textes/IMGTrepertoireRPI/2D-3Dstruct/representation3D/human/V-like/Hu_CD4Vdomain1.html last visited 2/15/05
Wu, H., Myszka, D. G., Tendian, S. W., Brouillette, C. G., Sweet, R. W., Chaiken, I. M., Hendrickson, W. A.: Kinetic and structural analysis of mutant CD4 receptors that are defective in HIV gp120 binding. Proc Natl Acad Sci U S A 93 pp. 15030 (1996)
Comments? Questions? Concerns? Please Email me at oshernandez@davidson.edu.