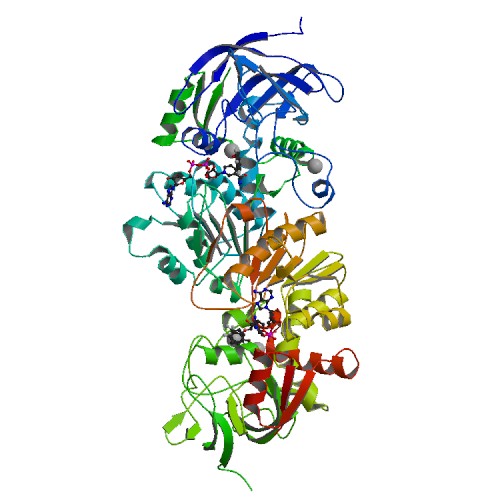
Figure 1. Structure of horse liver alcohol dehdryogenase complexed with NAD+ and substituted benzyl alcohols (Protein Data Bank)
Introduction
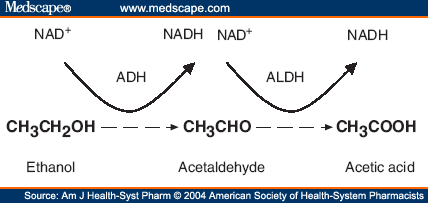
Alcohol dehydrogenase is an enzyme that is the main defense against alcohol in our bodies, allowing us to detoxify about one alcoholic beverage per hour (Fig. 1). Through oxidation of alcohol to acetaldehyde (which is later converted to an acetate by aldehyde dehydrogenase), ADH breaks down a toxic compound into molecules that can be utilized by our cells (Figure 2) (PDB).
Figure 2. Scheme of ADH mechanism using the example of ethanol. Permission pending Medscape
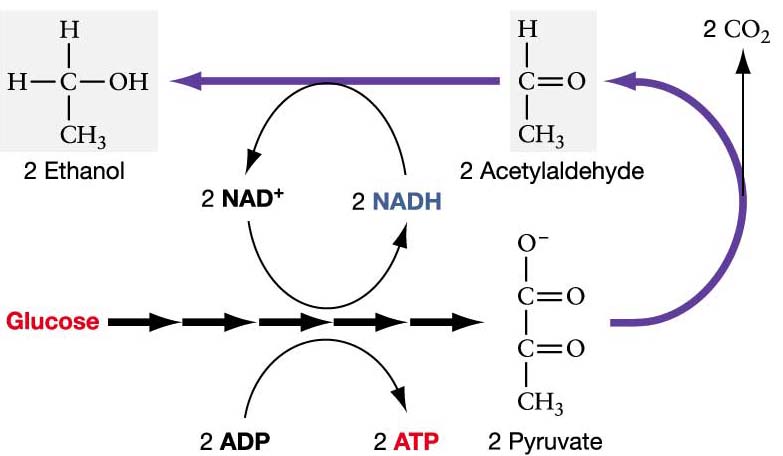
Humans create at least nine different isoenzymes that are encoded by seven different genes: ADH1, ADH2, ADH3, ADH4, ADH5, ADH6, and ADH7 (Davis et al., 1996). In yeast and bacteria, ADH also plays a major role in alcoholic fermentation. When a pyruvate is obtained as a product from glycolysis, it can be converted to acetaldehyde and carbon dioxde. ADH can then reduce acetaldehyde to ethanol, essentially reversing the process of alcohol oxidation (Figure 3).
Figure 3. Mechanism of alcohol fermentation. Permission pending mmuller@uic.edu
This page will focus on the structure and function of liver alcohol dehydrogenase (LADH), an ADH isoenzyme encoded by ADH1 (2).
Liver Alcohol Dehydrogenase Structure and Function Relationship
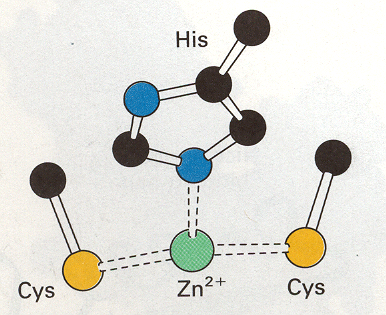
LADH is made of 374 amino acids and is a NAD-requiring enzyme that catalyzes the oxidation of primary, secondary, branched, and cyclic alcohols to aldehydes. The active enzyme has a molecular weight of 80,000 kDa and consists of two identical subunits that each contain a coenzyme binding site, a substrate-binding site, and two zinc atoms. The two subunits are connected by the interaction of their conenzyme-binding (NAD-binding) domains. One zinc ion is characterized as the "catalytic" or "active site" zince because it resides in the active site of the subunit and it also coordinates to the substrate (an alcohol) (1). Within the active site, this zinc ion is also coordinated to Cys-46, Cys-174, and His-67 (Figure 4) (Branden et al., 1973).This conformation keeps the zinc stable but also allows it to be accessible to the alcohol substrate.The other zinc atom is thought to play a structural role by maintaining the stability of the protein. While this zinc atom is bound near the surface of the enzyme (within one of the regions that joins the two subunits, near the coenzyme binding site), it is completely surrounded by four protein ligands (Cys-97, Cys-100, Cys-103 and Cys-111) and is not accessible to the alcohol. This zince atom is also 20 angstroms from the active site zinc.
Figure 4. Drawing of active site of LADH. Pending permission from the University of Wisconsin- Eau Claire.
In order for LADH to work, the coenzyme NAD+ must first bind to it. Then, the oxygen of the alcohol binds to LADH by coordination with the active site zinc. Binding to this electron-defecient site in the molecule increases the electron defecient character of the carbon atom on the alcohol, making it more likely that a substrate and not an inhibitor will bind to the active site. Furthermore, a hydrophobic pocket exists near the active site in which alcohols binding to LADH becomes dependent on. After binding to the active site, the alcohol is then deprotonated, leaving an alkoxide ion bound to zinc. A hydride transfer then occurs from the alkoxide ion to NAD+, which becomes NADH. This also produces an aldehyde or ketone bound to the zinc ion. Finally, both NADH and the resulting aldehyde/ketone dissociates from LADH (Hansch et al., 1971).
References
Hansch, C, Schaeffer, J, Kerley, R. 1971 November. Alcohol dehydrogenase structure-activity relationship. The Journal of Biological Chemistry; 274 (14): 4703-4710.
Branden, C, Eklund, H, Nordstrom, B, Boiwe, T, Soderlund, G, Zeppezauer, E, Ohlsson, I, Akeson, A.1973 August. Structure of liver alcohol dehydrogenase at 2.9-A Resolution. Biochemistry; 70 (8): 2439-2442.
Davis, G, Bosron, W, Stone, C, Owusu-Dekyi, K, Hurley, T. 1996 April. X-ray strucuture of Human B3B3 alcohol dehydrogenase. The Journal of Biological Chemistry; 271(29): 17057-17061.