Purpose: In this study, the researchers attempted to determine the factors sufficient for induction of pluripotent stem cells from mouse fibroblast cultures. Stem cells – before they differentiate – can become any kind of tissue. Unless differentiation is instigated, they can divide indefinitely as undifferentiated stem cells. After differentiation, however, the cell’s type is determined for the life of the cell – or so previously thought. In this paper, evidence is presented to suggest that, with addition of stem cell specific factors, differentiated cells can be returned to their pluripotent state in mouse embryonic and adult tissue cultures.
Background: They first determined genes that were up-regulated in Embryonic stem (ES) cells and/or cancer cells that play a part in maintenance of their pluripotency and longevity. 24 gene candidates were chosen for closer investigation as determinants for the ES cell type.
Verifying candidate factors:
They used homologous recombination to replace the FBx15 gene with a B-galactosidase gene fused to a neomycin resistance gene in MEF cultures. The FBx15 protein is specifically expressed in ES cells but unnecessary for maintenance of pluripotency or cell development. If this gene is even mildly expressed then it would provide resistance to G418. When MEF cultures were exposed to all 24 candidate ES-determining factors in the presence of G418, 22 colonies survived, meaning that the ES-specific FBx15 promoter had been activated. No single factor by itself caused activation of the FBx15 promoter, however, this proves that just these 24 genes are capable of inducing ES-specific gene expression.
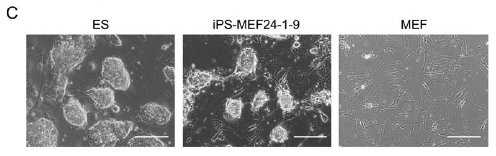
They called cells that showed ES-specific expression Induced Pluripotent Stem (iPS) cells. Five of these colonies showed ES cell-like morphology as opposed to MEF morphology (Figure 1C). They repeated the experiment and obtained 29 more colonies with G418 resistance, four of which showed ES cell-like morphology. The doubling time of these cells (called iPS-MEF24 for "induced pluripotent stem cells derived from MEF cells exposed to the 24 candidate genes") was more similar to ES cells than MEF cells and showed expression of known ES cell markers (Oct3/4, Nanog, E-Ras, Cripto, Dax1, Fgf4 and Zfp296). Bisulfite genomic sequencing of the promoter regions of Nanog, Fbx15 and Oct3/4 showed more methylation high similarity between Nanog and Fbx15 ES and iPS-MEF24 promoters. However, the iPS-MEF24 Oct3/4 promoter showed higher methylation pattern similarity with MEF cells than ES cells.

Narrowing Down candidate factors:
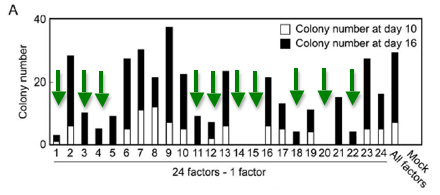
Next, they tried to determine which of these candidate factors were necessary for induction of the ES cell-type. By selectively removing a different candidate factor from 24 cell lines(Fbx15geo/Fbx15geo), they were once again able to determine which cells showed ES-like expression by growing them in media that contain G418.
As shown in Figure 2A, they found ten factors that--when removed--resulted in no colony growth 10 days after transduction of the other 23 factors and low to no cell growth after 16 days. This determined that the other 14 factors were not necessary for induction of ES cell expression patterns.
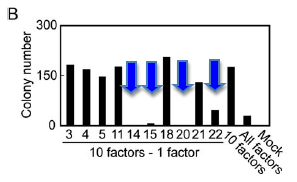
They then applied the same procedure to the remaining 10 factors to determine which of those genes were necessary for induction of ES cells (Figure 2B). The removal of either Oct3/4(factor 14) or Klf4 (factor 20) resulted in a lack of G814-resistant colonies. Removal of Sox2 (factor 15) resulted in a decreased number of colonies and removal of c-Myc (factor 22) caused the resulting colonies to have MEF-like morphology as opposed to the ES morphology shown by many of the other iPS cell lines.
Once again they applied the same assay to the four remaining genes to determine if any of them were unnecessary. Combinations of three out of the four genes could produce G418-resistant colonies but they were either short-lived or did not have proper ES cell morphology. Removal of Sox2 only resulted in viable cell lines resistant to G418 that had slightly different morphologies than iPS cells derived from all four factors (iPS-MEF4). They chose 6 clones from the iPS-MEF3 cell lines, which contained Klf4, c-Myc and Oct3/4 but not Sox2.
Evidence for ES cell induction:
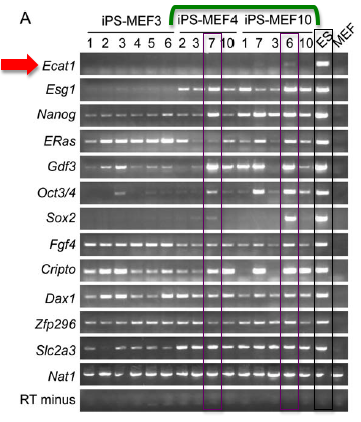
RT-PCR: Performing RT-PCR allowed them quantify the amount of mRNA that was produced from specific genes within the cell. Using primers specific for endogenous ES-specific genes, they performed RT-PCR analysis on iPS-MEF4, iPS-MEF10 and iPS-MEF3 cell lines (Figure 3A). iPS-MEF10 and iPS-MEF4 clones showed expression patterns that were remarkably similar to the ES control (when compared to MEF cells) except for Ecat1. The authors make note of Oct3/4 expression in iPS-MEF4-7, iPS-MEF10-6 and iPS-MEF10-7. In general, iPS-MEF10-6 cells appeared to very closely mirror ES cell expression patterns across nearly all of the genes.
Chromatin Immunoprecipitation and immunofluorescence: Chromatin immunoprecipitation analyses found increased acetylation of the histone H3 promoter and decreased dimethylation of lysine 9 of the histone H3 promoter when compared to ES cells (Figure 3B Not Shown). Methylation of CpG dinucleotides in these promoters also remained partially methylated in iPS cells. Increased methylation usually leads to decreased transcription which might cause the difference in expression when compared to ES cells. Staining with antibodies against SSEA-1 revealed that iPS-MEF10-6 and iPS-MEF4-7cells did produce SSEA-1 (stage-specific embryonic antigen 1), which is produced by undifferentiated early ES cells.
Microarray Data: The authors then used microarrays to gather data about ES, iPS and Fbx15geo/Fbx15geo MEFs (as a control) gene expression patterns. The microarracy data in Figure 4 (Not shown) shows that the iPS cells clustered together with the ES cells, forming two subclusters. One was formed of two iPS-MEF3 colonies and the other contained iPS-MEF10-6 and iPS-MEF4-7. The Fbx15geo/Fbx15geo MEFs clustered separately with MEFs expressing the four factors, immortalized MEFs expressing K-RasV12, and NIH 3T3 cells transformed by H-RasV12(************). They defined one group of genes in Figure 4B that is upregulated in both ES and iPS cells but not in MEF cells.
Despite the similarities in clustering patterns, the authors found several differences within iPS clones and between ES and iPS cells. One group of genes was found to be upregulated in iPS-MEF4-7 and iPS-MEF10-6 but not in either of the iPS-MEF3 clones. This suggests that exposure to three factors as opposed to four or ten leads to quantifiable differences in gene expression patterns. As will be discussed later, difference in regulation of this set of genes may lead to a nullipotent iPS phenotype. Furthermore, they found that iPS-MEF10-6 and iPS-MEF4-7 displayed downregulation of a third group of genes that were upregulated in ES cells.
Pluripotency Test: Even though the cells look like ES cells and have similar expression patterns, it is important to determine whether or not they actual possess pluripotent potential. In order to test this, they injected mice subcutaneously with cells from selected cell lines suspended in DMEM. They then waited four weeks for tumors to form, then dissected the tumors and performed histological analyses on the samples.
They obtained 5 iPS-MEF10 clones, 3 iPS-MEF4 clones, 1 iPS-MEF4wt clone, and 6 iPS-MEF3 clones. iPS-MEF10-6/3, iPS-MEF4-7/2 and iPS-MEF4wt clones produced tumors that contained all three germ layers. Figure 5A shows differentiation into Adipose, muscle, epithelial cartilage and CNS tissue. This demonstrates the ability of these cell lines to differentiate into multiple tissues. Some other clones were capable of differentiating into two germ layers but not all three. Unlike the other iPS clones, none of the iPS-MEF3 cell lines produced differentiated tumors, and were instead a mass of undifferentiated iPS cells. This data reveals that pluripotency is a characteristic of some but not all iPS cell lines and that three ES-inducing factors may be enough to create the semblance of an ES cell but are unable to recreate pluripotent capabilities.
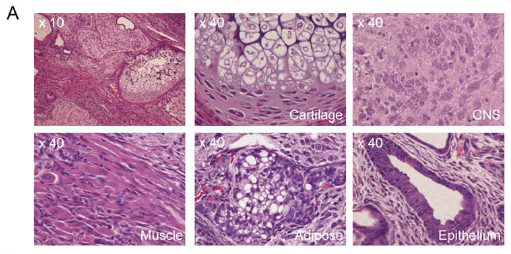
When true pluripotent stem cells are plated in noncoated, plastic dishes, they will adhere to the plate and begin to differentiate. Although this process is very similar to the formation of a true embryo, the cells' differentiation is disorganized and does not lead to formation of an embryo, but rather a mass of differentiated cells referred to as an embryoid body. When plated on noncoated dished, both iPS-MEF10 and iPS-MEF4 cells began the process of differentiation and formed embryoid bodies that tested positive for a-smooth muscle actin, a-fetoprotein and BIII tubulin (mesoderm, endoderm and ectoderm markers, respectively)(Figure 5D). Once again, iPS-MEF3 cells formed a mass that contained only undifferentiated cells. This solidifies the idea that iPS-MEF10 and iPS-MEF4 are pluripotent cell lines whereas iPS-MEF3 cells--while they are certainly similar to ES cells--do not have pluripotent capacity.
All these data suggest very real and significant similarities between iPS and ES cells, but they also illuminate the differences.
Induction of Pluripotency in Adult Fibroblasts:
Creation of iPS-TTF cell lines: In order to determine whether induction of pluripotency via transduction of their candidate factors was a phenomenon restricted to embryonic cells, they transduced their 4 final candidate factors into tail tip fibroblasts (TTF) from Fbx15/Bgeo homozygous 7-week-old male mice and one 12-week-old female mouse. The male TTF cells produced four G418 resistant colonies (iPS-TTF4). The same four-factor transduction procedure was applied to the female TTF cells, which constitutively expressed GFP due to prior transduction of the GFP transgene. 13 G418-resistant colonies were produced and 6 were selected to form 6 iPS-TTFgfp4 clones. A seventh iPS-TTFgfp4 clone was made using cDNA clones of the four factors which contained LoxP cut sites at either end of the factor-coding region. All of the iPS-TTF cell lines showed morphology identical to that of ES cells.
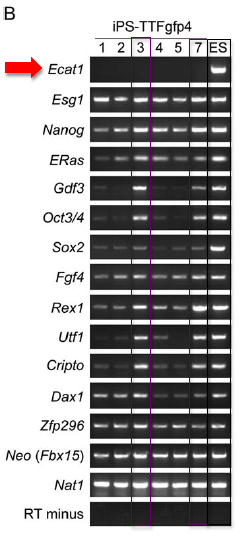
RT-PCR: They then used RT-PCR to determine which genes were expressed in iPS-TTFgfp4 cell lines (Figure 6A). Two cell lines (iPS-TTFgfp4-3 and iPS-TTFgfp4-7) showed a high degree of expression of ES-specific genes. All other iPS-TTFgfp4 cell lines showed varying degrees of similarity to ES gene expression.
Pluripotency Test: iPS-TTF4 and iPS-TTFgfp4 cells that were injected into nude mice produced tumors which contained all three germ layers, similar to both iPS-MEF10 and iPS-MEF6 cells.
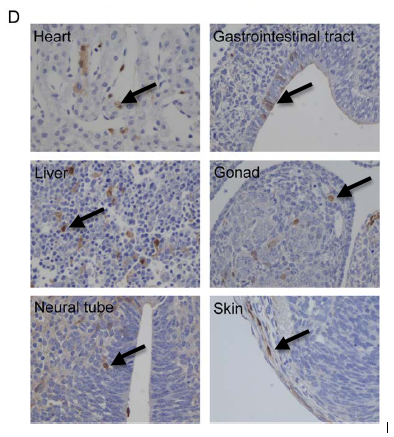
Localization of iPS-TTFgfp4 cells in developing blastocyst: Two iPS-TTFgfp4 clones (3 and 7) were injected into developing blastocysts. The embryos were then visualized at different stages of development and tested for the presence of GFP-producing cells. Both cell lines produced embryos that contained GFP-producing cells in all three germ layers (Figure 6D). This shows that iPS-TTFgfp4 cells were incorporated into all tissues of the developing embryo and were able to take the place of normal stem cells*. It also confirms the pluripotency of induced TTF stem cells.
Characterization of transgene expression and location: Western blots of the four factor proteins in iPS-MEF10-6, iPS-MEF4-7, iPS-TTFgfp4-3 and iPS-TTFgfp4-7cell lines showed that production of the four proteins was equivalent to production in ES cells (Figure 7A). Several other proteins that appeared at high levels in ES cells showed only moderate to low level production in iPS cells.
A southern blot (using a Klf4 probe) performed on a restriction enzyme digestion of DNA from several different iPS clones showed that the transgenes integrated at unique locations in each cell line. This may account for the differences between clones isolated from the same transduction processes. Finally, they determined that iPS cells, unlike ES cells, are unable to remain undifferentiated without feeder cells, even when cultured with LIF**.
The authors of this paper believe they have proven their method successful in inducing pluripotent stem cells from mouse fibroblasts. The iPS cells show gene expression and morphology that is very similar to ES cells while still maintaining MEF-like expression levels. This makes it less likely that the induced cells were some of the rare but possible stem cells found in muscle tissue, but rather the MEFs themselves. Some iPS cell lines are also pluripotent and capable of differentiating into all three germ layers. Others are not, suggesting that more than simple insertion into the genome is necessary for induction of pluripotency and that at least four of the necessary candidate factors must be present in order to facilitate pluripotency. The fact that evidence of iPS cells injected into blastocysts could be found in the developing embryos is clear evidence that the iPS cells are functioning at full pluripotent capacity among true stem cells.
Overall this paper was very well written. The figures were beautiful and well-organized and the writing was incredibly clear, given such a wealth of information and a potentially daunting (though exciting) topic. I think the evidence they provide is certainly adequate for the claims that they are making. They assume that their audience has quite a bit of prior knowledge about ES cells. Most of their background information about which genes are expressed, you have to simply accept or dig through their cited sources in order to find the evidence for each of the candidate factors. However, I have no reason to question their methods, they provide multiple pieces of evidence for each claim and their figures are very clean and compelling.
Having read the entire paper, I believe I would have liked to see the data that they included at the very end in the beginning of the paper. I realize that figures 1-6 present much more exciting material, but simply saying that you transduced MEF or TTF cells does not tell the reader much about the procedure. Knowing where and how the genes were included in the genome is potentially incredibly important to their conclusion. Disruption of another gene or unexpected expression patterns would render their study much less credible. Better to state those facts early on, rather that wait for Figure 7 at the very, very end of the paper to bring them up.
In general, they could have used a few more control images, especially in figures showing confirmation of germ layer differentiation. I don't believe that they are lying about the cell types that they found, bt it would be nice to have some kind of visual reference to compare it to.
*In this section, they go on to explain that none of the pups that were born were chimeric. They slip it in as a relatively innocuous point but this brings up some major questions. Did they see no chimeric mice, simply as luck of the draw? Or did the injected iPS-TTFgfp4 cells in some way disrupt the development of the embryo, such that a living pup was inviable? I would include this in my "Future Experiments" section as a high-priority topic of inquiry. This induction method has incredible potential, but only if the stem cells function properly once differentiated in a living, biological system. If the methods indicated in this paper can be applied to humans, then the implications are far-reaching and endlessly useful in the medical field. With parallel advancements in biotechnology, we may be able to create organs for transplant from a patient’s own tissue or be able to repair neuronal injury that the body cannot heal by itself, without the ethical concerns of human embryonic stem cell cultures.
My first instinct is to suggest that they begin trials on human cells immediately, but a short search determined that this was accomplished only a year after this paper was published (Nakagawa et al, 2007; Okita et al, 2007; Takahashi et al, 2007; Yu et al, 2007). After the initial outburst of papers in 2007, an onslaught of others appeared, making the same attempt with varying degrees of success.
As mentioned above, I think it would be beneficial to look into why no chimeric pups survived. If--after multiple attempts--chimeric mice fail to survive, further research into this phenomenon would certainly be warranted.
More directly related research would be to better characterize the differences between different iPS lines. Why do iPS-MEF10-6 and iPS-MEF4-7 show greater similarity to ES cells than other iPS-MEF10 and iPS-MEF4 clones? As noted in the paper, each clone has it's own unique integration pattern of the four genes transduced into the genome. Determining exactly where the transgenes inserted might be key to truly understanding how their method works. Using a plasmid rescue technique to fragment the inserted genes, you could transform bacterial colonies with the fragments of the genome, then purify DNA from the resulting colonies. Then run the DNA on a gel and probe the DNA for the transgenic sequences. Alternatively, they could use the known transgenic sequences as primers and use PCR to extract fragments of DNA which contain portions of the transgene and adjacent genomic DNA. It would then be a relatively simple task of matching the genomic fragments to known genomic sequences to determine the insertion site of each transgene.
The authors suggested in their discussion that the difference between iPS cell lines may be very slight differences in factor expression. If this is the case, then the next step would be to develop a way to moderate the expression of the transgenes that had been inserted into the genome. Perhaps, one could direct the insertion of transgenes into positions that had previously shown poor-ES morphology. If the transgenes were preceded by promoters that responded to varying degrees of some signal protein non-native to the cells, they could regulate the expression level of the gene and try to pin down the exact expression levels necessary for iPS formation.
In short, now that we have identified the factors necessary for induction of pluripotent stem cells, it is in our best interest to better understand how these factors function to create such drastic morphological changes. The better we understand them, the better we can control them and the faster we can start using the knowledge and skills we have gained to better society and the medical field.
** LIF (Leukemia Inhibitory Factor) is used in stem cell cultures to inhibit differentiation of stem cells. It is possible to culture ES cells without LIF by inducing overexpression of the gene Nanog (Williams, 1988).
Nakagawa, M. et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. Advance online publication 30 November 2007
Okita K., Ichisaka T., & Yamanaka S. (2007). Generation of germline-competent induced pluripotent stem cells. Nature 448, 260–262.
Yu, J, Maxim A. Vodyanik, Kim Smuga-Otto, Jessica Antosiewicz-Bourget, Jennifer L. Frane, Shulan Tian, Jeff Nie, Gudrun A. Jonsdottir, Victor Ruotti, Ron Stewart, Igor I. Slukvin, and James A. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science advance online publication.
Takahashi K. & Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–76.
Takahashi K. & Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872.
Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. (1988). Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 336, 684 - 687.