The purpose of this method is to separate proteins according to their size, and no other physical feature. In order to understand how this works, we have to understand the two halves of the name: SDS and PAGE.
Since we are trying to separate many different protein molecules of a variety of shapes and sizes, we first want to get them to be linear so that the proteins no longer have any secondary, tertiary or quaternary structure (i.e. we want them to have the same linear shape). Consider two proteins that are each 500 amino acids long but one is shaped like a closed umbrella whle the other one looks like an open umbrella. If you tried to run down the street with both of these molecules under your arms, which one would be more likely to slow you down, even though they weigh exactly the same? This analogy helps point out that not only the mass but also the shape of an object will detrmine how well it can move through and environment. So we need a way to convert all proteins to the same shape - we use SDS.
Figure 1. This cartoon depicts what happens to a protein (pink line) when it is incubated with the denaturing detergent SDS. The top portion of the figure shows a protein with negative and positive charges due to the charged R-groups of the particular amino acids in the protein. The large H represents hydrophobic domains where nonpolar R-groups have collected in an attept to get away from the polar water that surrounds the protein. The bottom portion shows that SDS can break up hydrophobic areas and coat proteins with many negative charges which overwhelms any positive charge in the protein due to positively charged R-groups. The resulting protein has been denatured by SDS (reduced to its primary structure) and as a result has been lenearized.
SDS (sodium dodecyl sulfate) is a detergent (soap) that can dissolve hydrophobic molecules but also has a negative charge (sulfATE) attached to it. Therefore, if a cell is incubated with SDS, the membranes will be dissolved and the proteins will be soluablized by the detergent, plus all the proteins will be covered with many negative charges. So a protein that started out like the one shown in the top part of figure 1 will be converted into the one shown in the bottom part of figure 1. The end result has two important features: 1) all proteins contain only primary structure and 2) all proteins have a large negative charge which means they will all migrate towards the positve pole when placed in an electric field. Now we are ready to focus on the second half - PAGE.
If the proteins are denatured and put into an electric field, they will all move towards the positive pole at the same rate, with no separation by size. So we need to put the proteins into an environment that will allow different sized proteins to move at different rates. The environment of choice is polyacrylamide, which is a polymer of acrylamide monomers. When this polymer is formed, it turns into a gel and we will use electricity to pull the proteins through the gel so the entire process is called polyacrylamide gel electrophoresis (PAGE). A polyacrylamide gel is not solid but is made of a laberynth of tunnels through a meshwork of fibers (figure 2 and figure 3).
Figure 2. This cartoon shows a slab of polyacrylamide (dark gray) with tunnels (different sized red rings with shading to depict depth) exposed on the edge. Notice that there are many different sizes of tunnels scattered randomly throughout the gel.
Figure 3. This is a top view of two selected tunnels (only two are shown for clarity of the diagram). These tunnels extend all the way through the gel, but they meander through the gel and do not go in straight lines. Notice the difference in diameter of the two tunnels.
Now we are ready to apply the mixture of denatured proteins to the gel and turn on the current (figure 4). If all the proteins enter the gel at the same time and have the same force pulling them towards the other end, which ones will be able to move through the gel faster? Think of the gel as a tiny forrest with many branches and twigs througout the forest but they form tunnels of different sizes. If we let children and adults run through this forest at the same time, who will be able to get through faster? The children of course. Why? Because of their small size, they are more easily able to move through the forest. Likewise, small molecules can manuver through the polyacrylamide forest faster than big molecules.
Figure 4. Cartoon showing a mixutre of denatured proteins (pink lines of differen lengths) beginning their journey through a polyacrylamide gel (gray slab with tunnels). An electric filed is established with the positive pole (red plus) at the far end and the negative pole (black minus) at the closer end. Since all the proteins have strong negative charges, they will all move in the direction the arrow is pointing (run to red).
You have to remember that when we work with proteins, we work with many copies of each kind of protein. As a result, the collection of proteins of any given size tend to move through the gel at the same rate, even if they do not take exactly the same tunnels to get through. Back to our analogy of the forest... If we were in a hot air ballon above the forest and watched 100 children, 100 teenagers, and 100 large adults running through the forest, we would see collection (or band) of children moving quickly, a band of teenagers moving slower, and a third band made of adults plodding their way through the forest. Likewise, proteins tend to move through a gel in bunches, or bands, since there are so many copies of each protein and they are al the same size. When running an SDS-PAGE, we never let the proteins electrophorese (run) so long that they actually reach the other side of the gel. We turn off the current and then stain the proteins (until we stain them, they are colorless and thus invisible) and see how far they moved through the gel. Figure 5 shows a cartoon gel and figre 6 shows a real one. Notice that the actual bands are equal in size, but the proteins within each band are of different sizes.
Figure 5. This shows a top view of an SDS PAGE after the current has been on for a while (positive pole at the bottom) and then turned off. The gel (gray box) has five numbered lanes where five different samples of proteins (many copies of each kind of protein) were applied to the gel. (Lane 1, molecular weight standards of known sizes; Lane 2, a mixture of three proteins of different sizes with a being the biggest and c being the smallest protein; Lane 3, protein a by itself; Lane 4, protein b by itself; Lane 5 protein c by itself.) Notice that each group of the three proteins migrated the same distance in the gel whether they were with other proteins (lane 2) or not (lanes 3-5). The molecular weight standards are used to measure the relative sizes of the unknow proteins (a, b, and c).
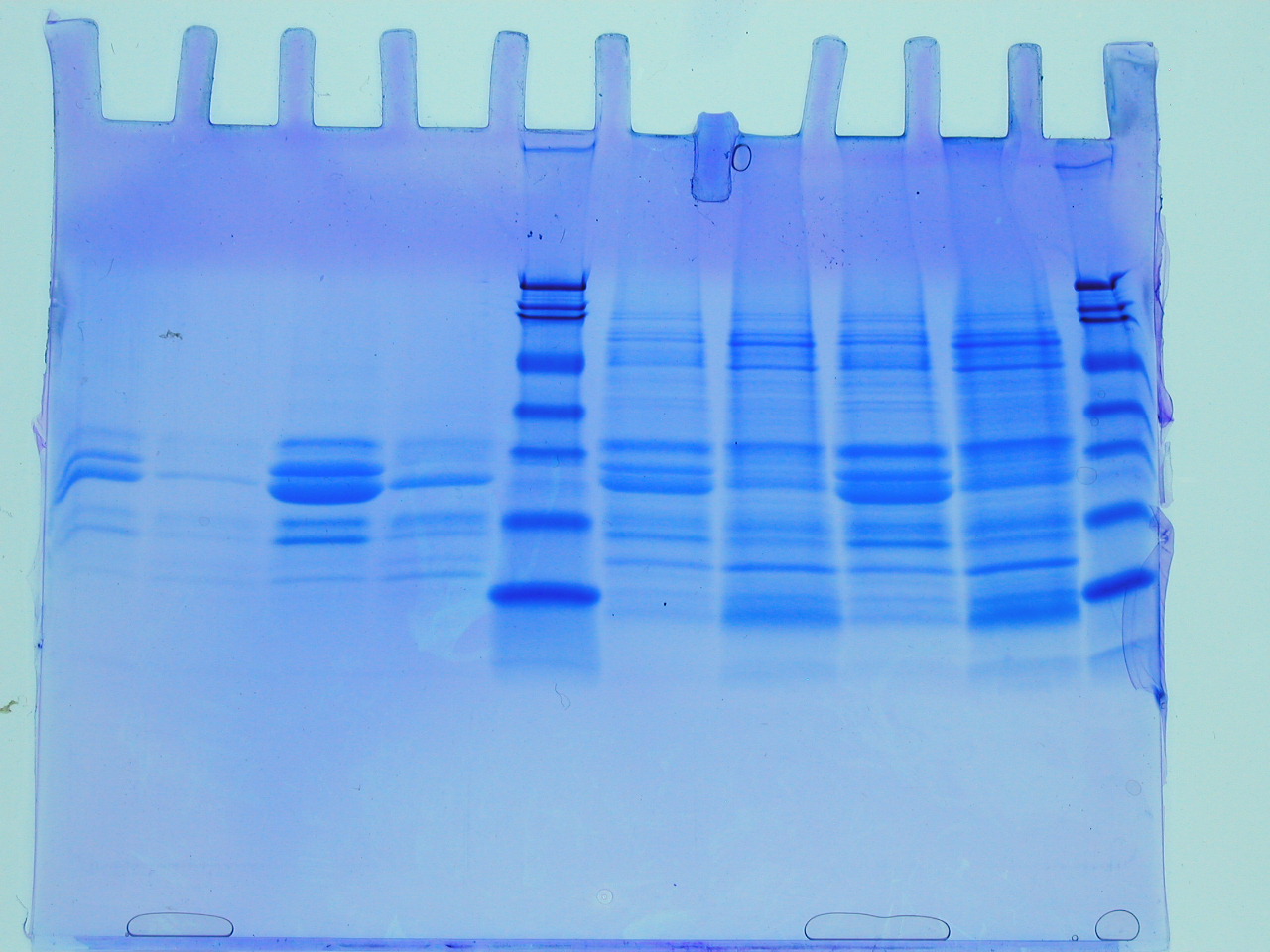
Figure 6. This photo shows a variety of different protein samples analyzed by SDS-PAGE. The blue dye allows you to see the accumulated molecules of varying sizes. Original figure from http://biology.ucsd.edu/classes/bicd123.SP07/