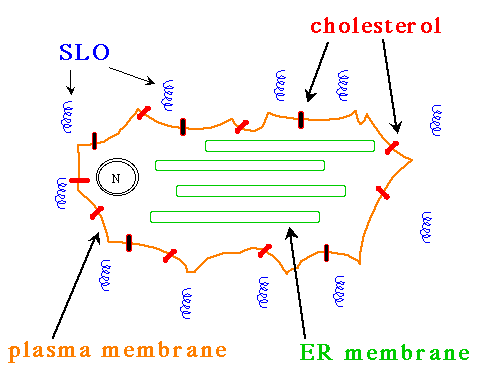
Streptolysin-O (SLO) is a bacterial protein that forms an alpha helix and is toxic to eukaryotic cells. Its toxic effect is due to the protein's ability to bind to cholesterol and form holes in the plasma membrane of cells. This ability can be utilized in the laboratory to create holes in the plasma membrane but no other membranes.
First, cells are incubated with SLO at 0° C and in the presence of calcium ions. Under these conditions, SLO will bind to cholesterol, but will not form pores. Since only the plasma membrane is exposed to the SLO monomers, no internal membranes are exposed to SLO and therefore will not be permeabilized by SLO.

Figure 1. Diagram of a eukaryotic cell with a high concentration of cholesterol (red bars) embedded in the plasma membrane (orange line). When incubated on ice in the presence of calcium ions, SLO (blue spirals) will bind to the cholesterol. Note that SLO does not bind to any other membranes within the cell.
The cells are then washed to get rid of excess SLO (that which is still in solution and not bound to cholesterol in the plasma membrane). Next, the cells are warmed to 37° C in the absence of calcium ions (EGTA, the calcium chelator, is added to the medium). Under these conditions, SLO forms multisubunit pores of approximately 13 nm in diameter.
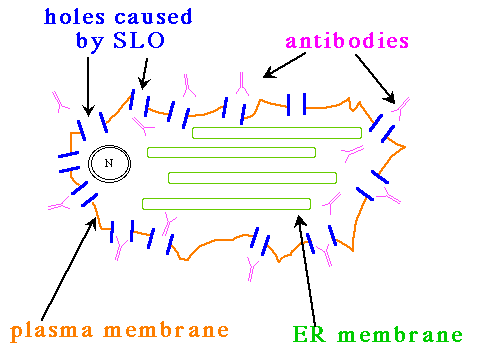
Figure 2. After the cells are warmed to 37° C and calcium is removed, the bound SLO monomers become embedded in the plasma membrane to create holes. Since there are no SLO monomers still in solution, none of the other membranes will be permeabilized by SLO; note that the ER and nuclear membranes are left unaffected.
The pores are large enough to allow proteins (e.g. anitbodies) to pass through, but not large enough for organelles. Finally, the cells are fixed with formaldehyde and processed for immunofluorescence microscopy.
Reference: Campbell, Kessler, and Fambrough. (1992) The Alternative Carboxyl Termini of Avian Cardiac and Brain Sarcoplasmic Reticulum/Endoplasmic Reticulum Ca2+-ATPases Are on Opposite Sides of the Membrane. Journal of Biological Chemistry. 267 (13): 9321-9325.
Return to Molecular Biology Main Page
© Copyright
2000 Department of Biology, Davidson College, Davidson, NC 28036
Send comments, questions, and suggestions to: macampbell@davidson.edu