Figure 1. Both figure
and figure legend reproduced with permission by Dr. John W. Kimball.

Figure 2. Both figure and figure legend courtesy of National Center for Biotechnology Information.
BCR-ABL:
CHRONIC MYELOID LEUKEMIA
What is Chronic Myeloid Leukemia?
Leukemia is a cancer of blood-forming tissues: the bone marrow, liver and spleen. It is characterized by the uncontrolled growth of blood cells. Chronic myelogenous leukemia develops in the white blood cells known as myeloid or granulocytic cells. The bone marrow gradually slows the production of normal blood cells and the person's myelogenous white blood cell count becomes elevated. The disease is considered chronic because it progresses slowly and may not cause any symptoms until late in the disease (Garl, 2001).
What are CML's Genetic Causes?
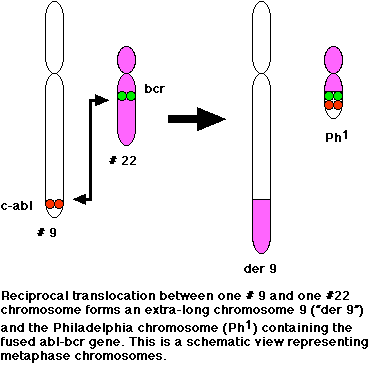
Chronic myeloid leukemia is not hereditary; it is caused by a change in the DNA of immature bone marrow cells. This change takes place on the Philadelphia chromosome, also known as chromosome 22. A reciprocal translocation between chromosome 9 and chromosome 22 occurs when pieces of each chromosome break off and switch with each other. The piece of chromosome 9 contains the gene c-ABL; the piece of chromosome 22 contains the gene BCR. Hence, the gene that is formed as a result is called the BCR-ABL gene. The new gene is responsible for producing a mutated protein which in turn converts the marrow stem cells from normal to leukemic. The resulting hybrid gene BCR-ABL codes for a fusion protein with tyrosine kinase activity, which activates signal transduction pathways, leading to uncontrolled cell growth (Kimball, 4 May 1999).
Cartoons illustrating the mutation that causes CML:

Figure 1. Both figure
and figure legend reproduced with permission by Dr. John W. Kimball.

Figure 2. Both figure and figure legend courtesy of National Center for Biotechnology Information.
So what does this mutated protein look like?
How does this mutated protein cause Chronic Myeloid Leukemia?
Tyrosine kinases are
a class of proteins that phosphorylate tyrosine residues. They can
be either receptor proteins, in which case they include an extracellular
ligand-binding domain, or they can be nonreceptor proteins with a cytoplasmic
domain. They often play essential roles in development and cell division
(Dow, 2000).
According to a recent
study published in the Journal of the American Medical Association, BCR-ABL
codes for a constitutively active cytoplasmic tyrosine kinase, meaning
the cells bypass regulated growth and undergo a malignant transformation
to leukemia. This particular tyrosine kinase can phosphorylate several
substrates resulting in the initiation of multiple signal cascades involved
in cell growth, differentiation, adhesion and death (Kalidas
et al., 2001).
According to recent news articles, a new wonder drug called Gleevec is a veritable "wonder drug."
CNN claims Gleevec is the wave of the future. A pill administered once a day with minimal side effects, Gleevec works by seeking out and destroying only cancer cells, while healthy cells remain virtually untouched. The pharmaceutical company Novartis boasts essential cures in a majority of patients tested (Rowland, 2001).
ABC News explains how Gleevec works: "Gleevec, formerly known as STI-571, can cause remission of cancer by inhibiting an enzyme produced by an abnormal gene called BCR-ABL that generates cancer cell growth" (Dunham, 2001).
According to Newsweek, Gleevec has few side effects and is so promising that cancer might one day soon be as treatable as, say, a bacterial infection (Kalb, 2001).
Time magazine claims that "these new medicines are like a troop of snipers, firing on cancer cells alone and targeting their weakest links...[they] could transform cancer from an intractable, frequently lethal illness to a chronic but manageable one akin to diabetes and high blood pressure" (Lemonick and Park, 2001).
So how does Gleevec really work? Is Gleevec the almighty cure for cancer?
According to Gleevec's producers, the drug is the first of its kind to target a specific molecule in the signaling transduction pathway. The medication inhibits the production of tyrosine kinase generated by the mutant BCR-ABL gene, thus keeping the overproduction of leukemic cells in check.
CML is one of the four most common types of leukemia, yet it only occurs in one to two cases per 100,000 people per year, accounting for 15-20% of all adult leukemias. Also, depending on when Gleevec is administered, patients may relapse. While no one can disagree that Gleevec is an extraordinarily promising treatment, because the drug is so new, we can hardly call it a cure (Novartis Press Release, 26 June 2001).
The scientific literature
agrees. According to the aforementioned JAMA paper, relatively specific
BCR-ABL
tyrosine kinase inhibitors were developed in 1996. STI-571, so named
for signal transduction inhibitor number 571 and marketed by the Switzerland-based
pharmaceutical company Novartis under the popular name Gleevec, "demonstrated
inhibition of proliferation of Bcr-Abl expressing cells as well as inhibition
of Bcr-Abl (+) tumor formation in profoundly immunodeficient mice."
STI-571 binds to the adenosine triphosphate–binding site of ABL,
thus inhibiting the phosphorylation of substrates and subsequent malignant
transformation to cancerous cells. Also, STI-571 is relatively ineffective
against other tyrosine kinases.
The authors of this
paper point out that there are hundreds of pathways through which CML can
develop; STI-571 targets only one of them. Therefore, further research
should be done to decide which, if any, other drugs may be helpful if used
in conjunction with STI-571 (Kalidas
et al., 2001).
A Few Conclusions:
1). Chronic myeloid leukemia is a unique disease in that it is caused by a single mutation creating a single gene that results in the overproduction of a single protein. Another definitive characteristic of the disease is how much knowledge we have of its pathogenesis. Compared to other diseases, few mysteries shroud the onset of CML.
2). The promising trials of Gleevec have many people overwhelmed with anticipation for finding a cure for cancer. As a treatment for CML, Gleevec may very well be a wonder drug in that it is easy to administer and has relatively few adverse side effects. However, it is important to remember that is also a very new drug. Long term effects, such as resistance and subsequent relapses, have yet to be established.
3). Gleevec has its limitations. It is designed to treat a specific molecular problem, whereas most cancers, along with most diseases in general, are not so simple.
4). CML constitutes a relatively
small number of cancer victims. More common cancers, e.g. lung cancer,
result from a combination of genetic and environmental factors and create
a host of molecular problems. In these cases, a simple solution is
infinitely more difficult to achieve.
References.
Dow J. 2000. Receptor Tyrosine
Kinase. <http://www.mblab.gla.ac.uk/~julian/dict2.cgi?5572>
Accessed 2001 Sept 11.
Dunham W. 2001 June 22.
Leukemia fights back. <http://abcnews.go.com/
sections/living/DailyNews/leukemia_gleevec010622.html>
Accessed 2001 Sept 2.
Garl S. Unknown date
of publication. Leukemia-CML. <http://oncology.com/ndg/
ndg_v2_MainFrame/1,3188,_12|00233|00_21|001|00_04|0040|00_18|003684|00_17|00231|
00_03|00312057|00_19|003690|00_20|001,00.html>
Accessed 2001 Sept 2.
Kalb C. 2001 May 28. A Cure
for Cancer? Newsweek. <http://archives1.newsbank.com/ar-search/
we/Archives?p_action=search&p_theme=NWEC&p_product=NWEC&p_perpage=20&s_search_type=
keyword&p_text_base=Glivec&p_sort=_rank_%3AD&xcal_ranksort=4&xcal_useweights=yes&p_field
_date-0=YMD_date&p_params_date-0=date%3AB%2CE&p_text_date-0=-1qzY&p_field_YMD_
date-0=YMD_date&p_field_YMD_date-0=YMD_date&p_params_YMD_date-0=date%3AB%2CE&%5B
+Search+%5D.x=54&%5B+Search+%5D.y=14>
Accessed 2001 Sept 2.
Kalidas M, Kantarjian H, Talpaz M. 2001. Chronic Myelogenous Leukemia. Journal of the American Medical Association 286(8). <http://jama.ama-assn.org/issues/v286n8/rfull/jct10003.html>Accessed 2001 Sept 7.
Kimball JW. 1999 May 4. Chronic Myelogenous Leukemia (CML). <http://www.ultranet.com/~jkimball/BiologyPages/C/CML.html> Accessed 2001 Sept 2.
Lemonick M and Park A. 2001. New Hope for Cancer. Time. 2001 May 28: 63-69.
Rowland R. 2001 March 1. Cancer
pill speeds through testing. <http://www.cnn.com/
2001/HEALTH/conditions/03/01/leukemia.drug/index.html>
Accessed 2001 Sept 2.
Novartis AG Press Release.
2001 June 26. Novartis' Leukemia Drug Glivec Approved in
Switzerland. <http://dominoext.novartis.com/NC/NCPRRE01.nsf/
8bd276aed5ee15e4412564f7005df22f/ded7da362a7d6e78c1256a7700324fd7/$FILE/
Glivec-App-CH-E.pdf>
Accessed 2001 Sept 2.
Department of Biology, Davidson College, Davidson, NC 28036
Send
comments, questions, and suggestions to: emoldham@davidson.edu