A recent study conducted by the Cardiff University’s School of Medicine has found a genetic basis for Attention Deficit Hyperactive Disorder (ADHD). The Center of Neuropsychiatric Genetics and Genomic Department of Psychological Medicine and Neurology performed searched the genome for deletions and duplications at given loci, called Copy Number Variants (CNVs). The researchers claim that CNVs are known to cause neurological disorders similar to ADHD. In their work, they hoped to address the following concerns:
They recruited 410 children from child psychiatry and community clinics in the United Kingdom who had a clinical diagnosis of ADHD and compared the genomic analyses with those of 1156 ethnically related matched controls. The findings were significant, concluding that the genomes of children with ADHD contained 2.09 times more CNVs than those who did not (p=8.9 x 10-5). In addition, they found that of the CNVs identified those with ADHD, nine of 40 overlapped with predetermined loci for schizophrenia (Williams et al., 2010).
These results were published in a late October 2010 issue of The Lancet and consequently spurred a handful of articles in the popular press, namely USA Today and the Psychiatric News. Compared to the The Lancet, USA Today is meant for a non-science specific audience, while Psychiatric News is meant for an interest specific audience that is more familiar with neurological terminology. Popular press articles tend to provide a succinct summary of the results presented in a scientific study using terms easily understood by the general public. According to Kua et al., two major problems that weaken reporting of scientific studies in the popular press include the lack of information— mainly due to overlooking methodology—and the lack of context (Kua et al., 2004). They further attempt to oversimplify issues to maintain the interest of the lay reader. The following analysis provides an overview of the study conducted by Williams et al. and then compares and contrasts the popular press’ reporting of this new finding with the results published in The Lancet.
Williams et al. begin by acknowledging the mechanism of ADHD’s long-suspected heritability are still unknown; however, evidence points towards CNVs as a possible cause or effect of ADHD (Williams et al., 2010). In their study, they investigate whether ADHD diagnosed individuals were prone to CNVs in comparison with the control and whether these CNVs also correlated with an associated intellectual disabilities. They selected 410 children from 90 community child psychiatry and pediatric clinics who were diagnosed with ADHD via the Diagnostic and Statistical Manual of Mental Disorders (DMS-IV) or International Classification of Diseases (ICD)-10 criteria. Additionally, symptoms of social and intellectual impairment at school were confirmed by each of their teachers. Of these children, 91.5% were confirmed single cases of ADHD (no affected siblings).
Selecting Individuals for the Study
In order to account for the fact that CNVs are known to occur in patients with higher intellectual disabilities, Williams et al. used the Wechsler Intelligence Scale for Children-IV to test for intellectual disabilities. Children with an IQ below 70 were diagnosed with intellectual disabilities. The study was also replicated in an Icelandic population, with participants chosen in a similar fashion. Neither study included schizophrenic or autistic individuals as part of the sample.
Methodology
In order to find where the genomes needed to be analyzed, Williams et al. genotyped each of the ADHD patients to find single nucleotide polymorphisms (SNPs). SNPs are the mutation of a single nucleotide (ie, adenine to cytosine) and are the most common genotypic variation in people. They occur about every 300 base pairs (bp) in the genome (Huentelman, 2005). If scientists can mark the location of every SNP in an individual, they can deduce both the inheritance patterns within families and the location of disease-specific genes. In fact, SNPs are efficient markers for genome-wide linkage scans, which was the method of choice for this study by Williams et al. (Huentelman, 2005).
The genomes had to meet specific criteria in terms of the frequency and location of SNPs in order to qualify for the study. In the end, they selected 366 eligible patients with ADHD and 1047 controls for full analyses. In these remaining patients, they performed a “CNV call” analysis. A “call” is a way to indicate the genotype of an organism. For example, SNP genotyping or “SNP calling” determines which base has replaced another base at a given position in the genome (Netterwald, 2008). The team only decided to observe CNVs larger than 500 kb because these give the highest call accuracy and congruent predictions on all levels. They also further assessed the size of the CNV using Agilent Human Genome comparative genomic hybridization 44K microarrays whose probes were designed to signal containing CNVs larger than 500kb (Williams et al., 2010).
Results & Analysis
Following the exclusion of the most common CNVs, each of the 366 patients analyzed contained at least one of the 135 remaining rare CNVs larger than 500 kb. Table 1 depicts the number of CNVs that duplications and deletions in the control and variant groups. If there were CNVs of different categories that overlapped, they were combined into a single locus that encompassed all of those contiguous CNVs. Williams et al. also attempted to pinpoint CNVs containing predetermined loci for schizophrenia and autism. The only locus causally linked to both schizophrenia and autism was on chromosome 16. The thirteen other combined locations for these two diseases were all independent of each other (Williams et al., 2010).
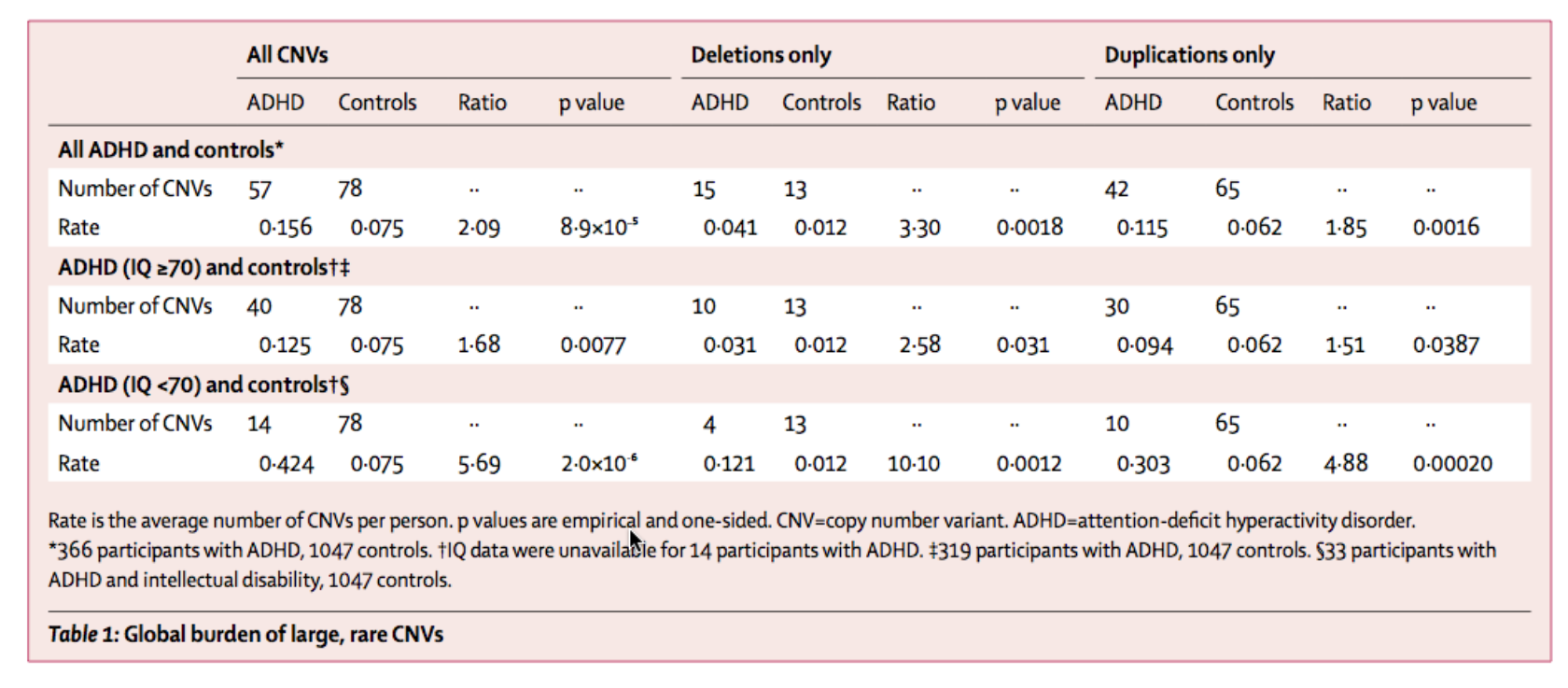
Image courtesy of Williams et al., 2010
At the end of the study, 14% of children with ADHD contained a CNV larger than 500 kb compared with 7% of the control sample. Williams et al. also found that 36% of children who had an intellectual disability in addition to ADHD contained a CNV larger than 500 kb, while only 11% of children with ADHD and no intellectual disability contained enlarged CNVs. When observing only children with ADHD and no intellectual disabilities, the researchers found that there were eight of 40 CNVs and nine of 40 CNVs overlapped loci previously associated with autism and schizophrenia respectively. Later analysis showed that the small arm of chromosome 16 in children with ADHD contained an excess of CNVs; this was primarily caused by duplications spanning an 824 base consensus region. This data was successfully replicated in an Icelandic population of 825 patients with ADHD.
The study concludes that an excess of rare CNVs that include both duplications and deletions is a strong factor behind ADHD diagnosis; however, genome wide association and linkage studies do not yet indicate the common genetic risk variants. Although schizophrenia and ADHD are thought to be separate disorders, ADHD and autism are suspected to have a “shared biological basis” because they have similar clinical symptoms (Williams et al., 2010). At the same time, findings of the large, rare CNVs in patients with autism, intellectual disability, and schizophrenia suggest that they factors into diverse neurodevelopmental disorders. The research team emphasizes the need to further understand the role that CNVs play in an ADHD diagnosis.
One of the contributors, Dr. Anita Thapar, also helped publish a study on the comorbidity of ADHD behaviors using the twin study method in 2001. Another of Dr. Thapar's studies in 2010 discusses how the prevalence of ADHD correlates with a variable number tandem repeat in the Dopamine D5 receptor (DRD5), dopmine D4 receptor (DRD4), and dopamine transporter (DAT1). Both pieces of literature are helpful supplements to the discoveries made regarding CNVs.
The article published in the November issue of Psychiatric News is based off the study conducted by Williams et al. Compared to the original scientific article, this popular press article focuses on an audience whose interests lie in the neurological field; therefore, the intended audience is more general, and not as “versed” in genomics compared to audience of The Lancet. Aaron Levin begins with a general description of the study, stating that it found children with ADHD to have “more large genetic variations than controls” (Levin, 2010). He also capitalizes on the commonly held perceptions of ADHD (only to weaken it) by including Anita Thapar’s comment on the disease being based solely in poor parenting and diet.
Levin’s article contains both strengths and weaknesses. In accordance with the standards of scientific communication set by Kua et al., Levin proficiently places the study in an understandable context while highlighting methodology (Kua et al., 2004). For example, he accentuates the importance of relating the study to known diseases such as schizophrenia and autism and provides both a definition and an explanation of the study’s central focus on CNVs.
Conversely, the remainder of his article appears to be a summary rather than an explanation of the study’s results. He fails to expound upon the borrowed figures and fragments from the scientific literature and masks the latter half of the article with facts, quotes, and numbers. While Williams et al. only briefly mention the possibility of two genes (NDE1 and DISC1) that are linked to ADHD, Levin chooses to include these in his article as further support for the association with schizophrenia. He quotes a doctor in the United States not involved in the research efforts to indicate that Williams et al. support previous findings of ADHD’s biological basis, without specifying what these findings exactly are. Extending the vague conclusion, Levin informs the readers that current studies do not provide sufficient evidence for the mechanism of ADHD development and future research and more funding are needed to “illuminate the biology of these genes”. Overall, his article contains a stronger introduction to the research study, but culminates with a repetitive and vague conclusion.
While Psychiatric News and The Lancet rely on science-specific audiences, USA Today targets a very general, non-science specific audience. Immediately, the article begins with a grave exaggeration and asserts that “children with [ADHD] are twice as likely to have a missing or extra chromosome than other children.” In addition to misunderstanding the basic definition of Copy Number Variants, USA Today makes a claim that Williams et al. do not make in their study, namely that children are “twice as likely”. This mistake is a perfect model of Kua et al. describes as a difference in what is said, rather than how it is said (Kua et al., 2004).
The article mentions the demographics involved in the study with less detail than Psychiatry News. Additionally, USA Today is less specific in its methodology. It hones in only on the aspect of the study that dealt with links to schizophrenia and autism. The article does mention the difference in deletion and duplication frequencies between control and ADHD, but hastily uses them in context of the entire chromosome. It also fails to provide the readers with a term that would encompass both deletions and duplications: CNV.
While the lack of great detail is obvious in USA Today’s article, there are certain features of the article that contribute to its public value. The article is a quick and easy read, and provides factual bits of information that are easy to remember, but ultimately do not contribute to an organized passage. Explanations of the study are vaguer than either of the science-specific articles; however, Kua et al. explains that a shift from logos to pathos occurs in popular press articles with a scientific scope who do not want to “scare away” their lay readers with unfamiliar jargon (Kua et al., 2004). Such is evident in USA Today’s article. Halfway through the passage, the article's authors appeal to the pathos of their readers by including a segment on ADHD’s worldwide incidence in children to imply that the need for research in this field is dire.
The conclusion of this article includes many ambiguous quotes that are overshadowed by the prestigious titles of the individuals from whom they come. For example, a professor at of molecular neuroscience at the University of Burbach states that ADHD is “the last phase of development that’s gone wrong” and that “the brain just needs to be fine-tuned.” In light of these statements, Kua et al. would encourage us to ask, What developments have gone wrong? In what sense does the brain need to be “fine-tuned”? Can we really cure ADHD so simply (Kua et al., 2004)?
Take me back to Shamita's Homepage.