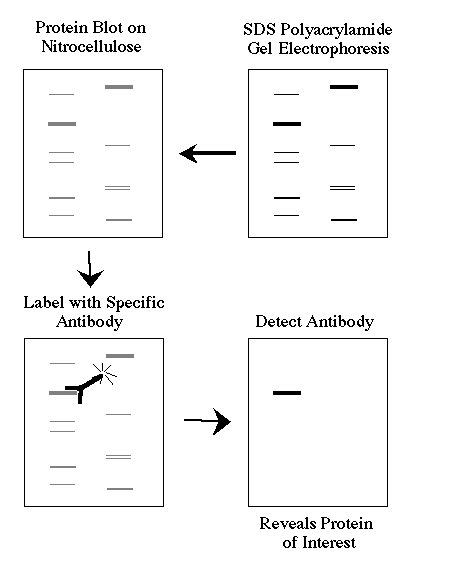
This is a brief overview of how a western blot (more formally called a protein immunoblot) is performed and what type of data you can obtain from one.

Western blots allow investigators to determine the molecular weight of a protein and to measure relative amounts of the protein present in different samples.
1) Proteins are separated by gel electrophoresis, usually SDS-PAGE.
2) The proteins are transfered to a sheet of special blotting paper called nitrocellulose, though other types of paper, or membranes, can be used. The proteins retain the same pattern of separation they had on the gel.
3) The blot is incubated with a generic protein (such as milk proteins) to bind to any remaining sticky places on the nitrocellulose. An antibody is then added to the solution which is able to bind to its specific protein. The antibody has an enzyme (e.g. alkaline phosphatase or horseradish peroxidase) or dye attached to it which cannot be seen at this time.
4) The location of the antibody is revealed by incubating it with a colorless substrate that the attached enzyme converts to a colored product that can be seen and photographed.
© Copyright 2001 Department of Biology, Davidson College, Davidson, NC 28036
Send comments, questions, and suggestions to: macampbell@davidson.edu