This web page was produced as an assignment for an undergraduate course at Davidson College.
Janet G. M. Markle,1,2 Daniel N. Frank,3 Steven Mortin-Toth,1 Charles E. Robertson,4 Leah M. Feazel,3 Ulrike Rolle-Kampczyk,5 Martin von Bergen,1,6,7 Kathy D. McCoy,8Andrew J. Macpherson,8 Jayne S. Danska1,2,9*
1Program in Genetics and Genome Biology, Hospital for Sick
Children Research Institute, Toronto, Ontario M5G 1X8, Canada. 2Department of Immunology, University of Toronto,
Toronto, Ontario M5S 1A8, Canada. 3Division of Infectious
Diseases, University of Colorado School of Medicine, Aurora,
CO 80045, USA. 4Department of Molecular, Cellular, and Developmental
Biology, University of Colorado, Boulder, CO
80309, USA. 5Department of Metabolomics, Helmholtz Center
for Environmental Research, 04318 Leipzig, Germany. 6Department
of Proteomics, Helmholtz Center for Environmental
Research, 04318 Leipzig, Germany. 7Department of Biotechnology,
Chemistry and Environmental Engineering, Aalborg
University, 9000 Aalborg, Denmark. 8Maurice Müller Laboratories,
Universitätsklinik für Viszerale Chirurgie und Medizin
(UVCM), University of Bern, 3008 Bern, Switzerland. 9Department
of Medical Biophysics, University of Toronto, Toronto,
Ontario M5G 2M9, Canada.
*To whom correspondence should be addressed. E-mail:
jayne.danska@sickkids.ca
Like many other autoimmune diseases, Type I Diabetes (T1D) has a strong female bias. Lower testosterone levels have been shown to correlate with higher disease incidence, while microbial colonization has been demonstrated to effect to the opposite. Markle et al. colonized nonobese diabetic (NOD) mice of both genders suffering from T1D with commensal microbes in germ-free (GF) and specific pathogen-free (SPF) conditions, and report gender-specific gut microbiota that decrease or increase testosterone levels in female or male mature mice, respectively. Then, SPF-colonized female NOD weanlings were gavaged with diluted cecal contents from mature males, which altered their microbiota and metabotype to a state “distinct from both unmanipulated males and females” for more than 11 weeks, during which the mice displayed elevated testosterone levels with no change in fertility. Gavaging of females with male microbiota conferred insulitis protection, decreased anti-insulin antibodies and delayed autoreactive T cell activity, all of which were abrogated upon androgen receptor (AR) blockage, thereby demonstrating that attenuation of the autoimmune response was indeed caused by the increase in testosterone levels.
I was very excited to read this paper because of what I think is the potential for a very high impact of the findings reported by it. It strikes me as interesting how sexual dimorphism has been largely overlooked in autoimmunity research, leading to the publication of a paper with an idea as simple as tracking the effects of sex hormone levels in microbial transplantations as late as 2013. Therefore, I think that the findings of Markle et al. are likely to inspire a lot of future research in that area and possibly push the field of autoimmunity research, which has been struggling to think of therapeutic methods other than bone marrow transplantation, into an entirely new direction. It would definitely be interesting to see the effects of sex differences in the gut microbiome on all other autoimmune disorders and even conditions of immunodeficiency and hypersensitivity too.
Yet even though I am convinced by the argument that the researchers are making, I think their claims could have been substantially increased with just a little bit more effort. An additional experiment that I wish I could see the results of would be whether or not the induction of even higher testosterone levels in males (maybe via microbial transplantation as well) might not confer a yet better protection from T1D compared to in unmanipulated mature males. If that is so, microbial transplantations could potentially serve to effectively cure T1D instead of to only eliminate the sexual dimorphism in its prevalence.
Also, in addition to the metabolome comparison, I would also like to see a principle component analysis (PCA) of the bacterial 16S rRNA sequences of male to female recipients and female to female recipients compared to that of untreated males and females through the progression of developmental time. This could possibly identify particular species of commensal bacteria that are most responsible for the observed differences and therefore serve to pinpoint terapeutic targets.
Lastly, I find it a little disheartening that, as the authors claim, “human T1D is not sex-biased, perhaps because the peak age at onset precedes puberty”, which unfortunately diminishes the prevention perspectives suggested by the revelations of this paper. Luckily, the possibility remains that microbial transplants can be of therapeutic value, which I find more exciting than the direct administration of testosterone or AR agonists due to the long-term sustained effect as demonstrated by Markle et al. Nevertheless, I cannot help but wonder why is it that sexual dimorphism in autoimmunity exists and evolution has selected for females to have a higher tolerizing threshold than males, and, additionally, whether the reason for the existence of that threshold might not be one too important to justify its abrogation by such a treatment. Finally, I wonder whether or not, unlike in mice, the possible side effects of elevated testosterone levels in women might not prove too big of an obstacle for the commercial success of such method of treatment.

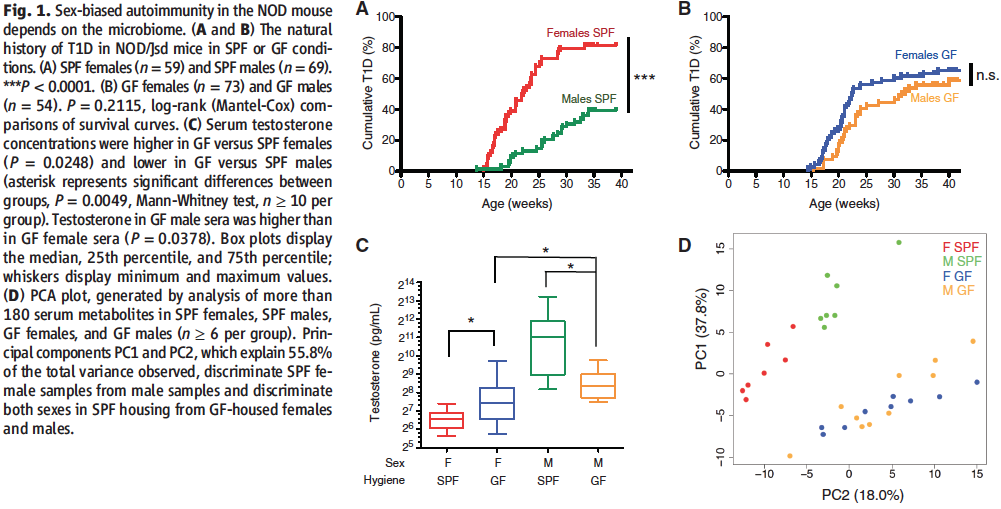
The first figure of the paper serves to confirm what is already known about the sexual dimorphism in T1D and relate it to the researcher’s experimental plan on investigating the influence of gut microbiota on autoimmunity.
In order to do that, Markle et al. start by inspecting whether commensal microbes have any influence on the likelihood of developing T1D in NOD mice in the first place. To answer this question, the researchers compared disease prevalence in male and female NOD mice separately in SPF or GF conditions (panels A and B, respectively). As it can be inferred from panel A (which can be regarded as control), sexual dimorphism does exist under regular conditions (which don’t allow the mice to develop another disease, however) and females are about twice as likely to develop T1D than males (peak ~80% vs. peak ~40%). This difference appears to be abolished in panel B where, under conditions restrictive of the development of any microbiota whatsoever, females have a lower prevalence and males have a higher prevalence of the disease compared to control and are relatively equivalent to each other (peaks ~60% for both).
In order to arrive at a more proximal cause for this phenomenon, the researchers then correlated the observations from panels A and B to data on changes in testosterone levels in between all of the same experimental conditions. As demonstrated by panel C, and in parallel with the data previously presented, females in an SPF environment have less testosterone and males in an SPF environment have more as compared to their GF equivalents. Therefore, it can be concluded that testosterone level fluctuations appear to be governed by colonization by commensal microbes as well as that higher testosterone levels appear to convey protection to T1D and vice versa.
In panel D, the researchers confirm that the differences in testosterone levels are attributable to distinct microbial communities by performing a PCA of the gut metabolomes of the mice via mass spectrometry. For GF males and females, where T1D prevalence and testosterone levels are similar (as evident from panels B and C), the metabolites were also similar (as evident from the lack of separation between the blue and yellow circles into 2 different clusters) and therefore it can be concluded that any metabolic differences in between the genders have to be due to the development of distinct microbial communities. Sure enough, this hypothesis is confirmed by the separation of SPF female and male metabolites (as represented by the red and green clusters, respectively). Importantly, both of these metabolite clusters are also distinct from those of the GF mice, thereby confirming that it is the microbial colonizations that cause the metabolome changes too.
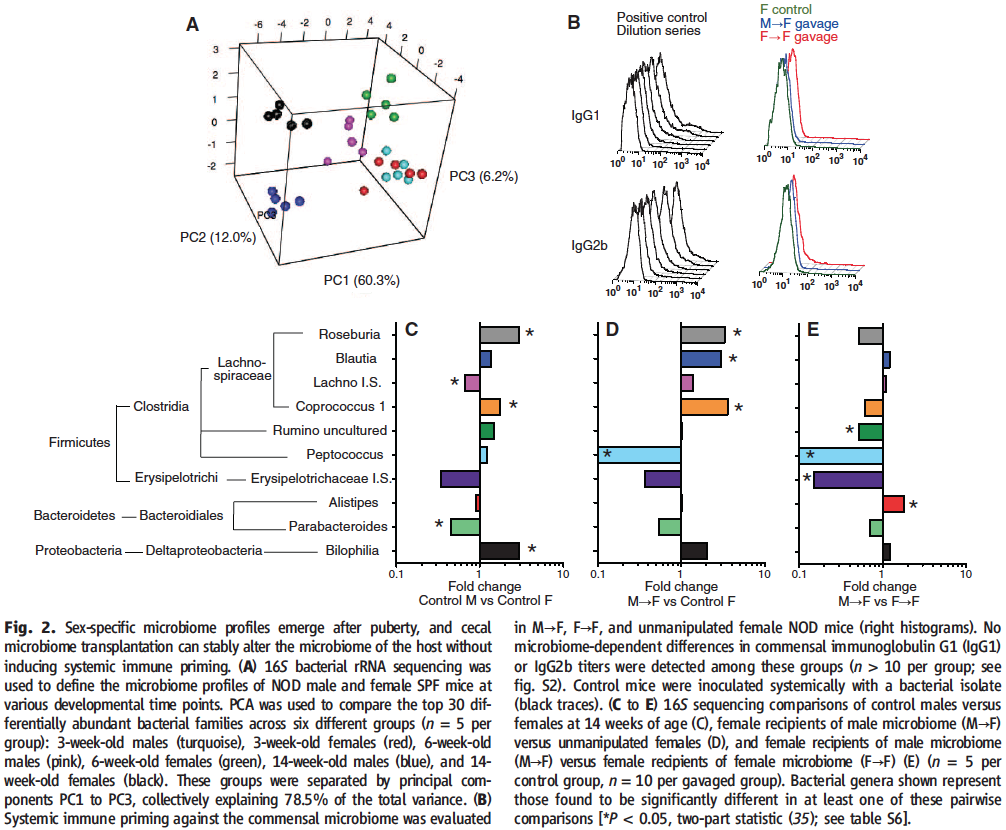
After characterizing the differences amounting to the sexual dimorphism observed in T1D in Figure 1, the researchers next wondered whether they might not be able to prevent the development of the disease in females by transplanting them with male gut microbiota.
To do that, in Panel A of Figure 2, Markle et al. first characterized the speciation of male and female gut microbiome profiles through their maturation by comparing 16S bacterial rRNA sequences for NOD mice raised in PSF conditions at different stages of their development. As evident from the increasingly more separated clustering of sequences into their respective experimental group (rRNA sequence clusters appear undifferentiated for 3-week-old mice and markedly gender-specific for 14-week-old mice), sex-specific microbiome profiles only emerge after puberty (6-weel-old mice) when the first prominent differences in sex hormone levels occur.
The researchers therefore decided that in order to achieve their goals, they would gavage female weanlings with diluted cecal contents from adult male donors (and from adult female donors for control). In order to make sure that the transplantation is not rejected by the host, in panel B the investigators check to see whether systemic immune priming was not induced in the weanlings against the transplanted bacteria. This possibility is eliminated as evident from the absence of increased IgG1 or IgG2b levels upon either male to female or female to female microbial transplantation (in comparison to the positive control).
In order to evaluate the effect of the transplantations, in panels C through E 16S rRNA sequences were compared in between transplant recipients and age-matched unmanipulated mice. As evident from all of the statistically significant variation in either of the pairwise comparisons, the suspected differences in microbiota were confirmed and it can be concluded that the male to female transplants resulted in the development of a microbiota distinct from that of unmanipulated females or males.
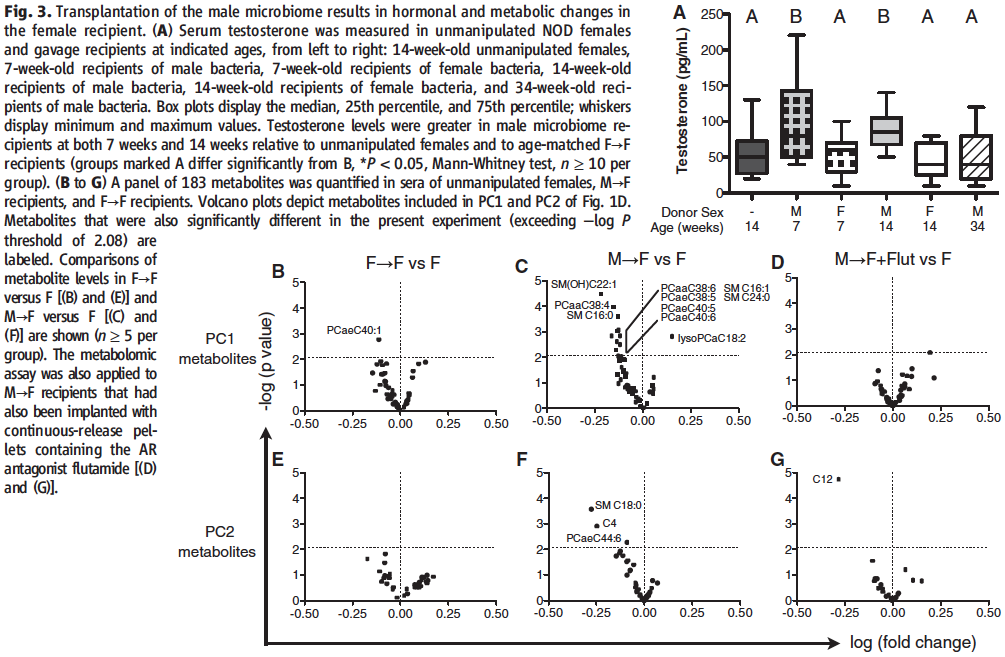
The researchers then looked into the question of whether the male to female transplantations induced the predicted elevated testosterone levels and different metabolites.
In panel A of Figure 3, serum testosterone levels were quantified in between transplants from either female or male mature mice donors into female weanlings at ages of 7, 14, and 34 weeks. As expected, none of the female to female transplants induced any testosterone change (testosterone levels remained around 50 pg/mL regardless of age), however, in direct comparison to them for the different developmental stages of the recipient mice, the male to female transplants induced significantly higher testosterone levels in 7-week-old and 14-week-old mice (~75 pg/mL), but not 34-week-old mice (~50 pg/mL). Importantly, this shows that the induced elevated testosterone production in a microbial transplant can be sustained over time. Of note, the normalization at age of 34 weeks also nicely corresponds to a previously reported decline in sexual dimorphism at that age for multiple sclerosis and rheumatoid arthritis.
In panels B through G of the figure, the researchers confirm that metabolome differentiation is present only in male to female transplants by performing a metabolite PCA focusing on the gender-specific metabolites characterized in Fig.1. This is made evident by the fact that the production of only one of the female to female metabolites (panels B and E) proved to be significantly different, in comparison to many more in the male to female transplants. Importantly, this experiment also proves that the metabolome differences were indeed caused by the induced change in testosterone levels, as evident from the fact that in panels D and G AR antagonism with flutamide was able to negate the production of almost all of the metabolites that were significantly induced by the male microbiome transplants in the neighboring panels.
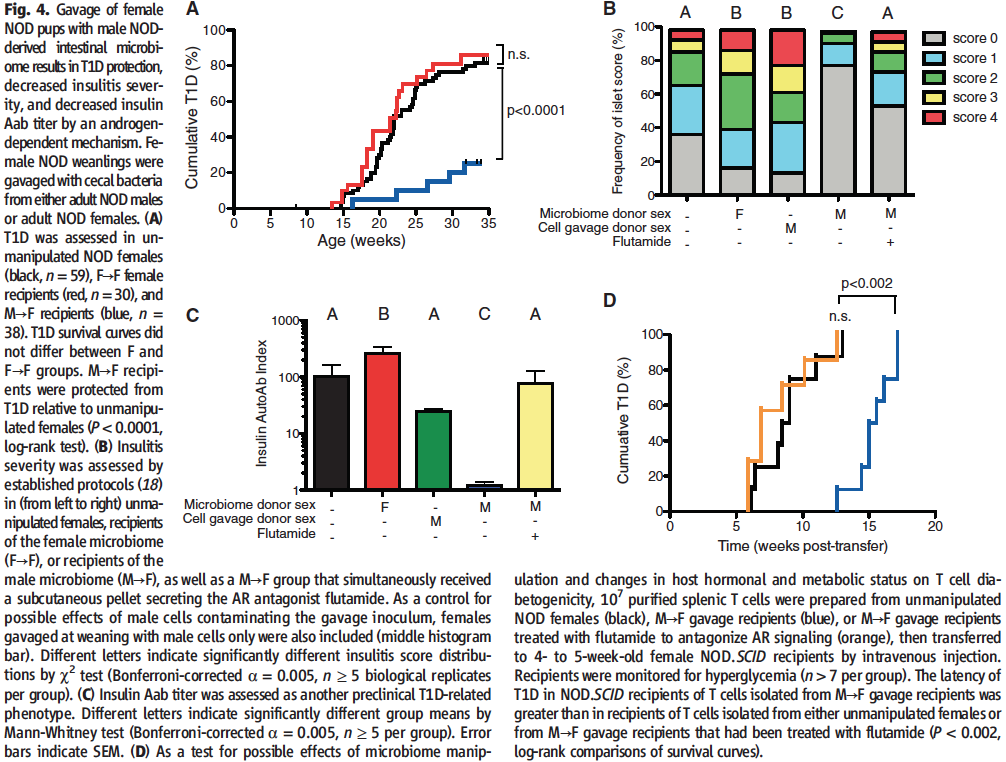
Finally, in Figure 4 Markle et al., set on to find out whether their male to female microbiota transplants had the hypothesized effect on moderating autoimmunity via several independent ways.
To investigate whether the microbial transfers were able to confer T1D protection, in panel A the researchers compared disease prevalence in between unmanipulated females, females that had received a microbial transplant from mature females and females that had received a microbial transplant from mature male NOD mice. As expected, the male to female transplants and them only were able to significantly decrease the prevalence of T1D in comparison to the other two subject groups (peak of ~25% vs. peak of ~80%, respectively).
In order to look into a more proximal effect of the microbial transfers, in panel B the researchers assessed insulitis severity in between the different transplant groups. As evident from the fact that almost 80% of the females that received male microbes failed to develop insulitis (score of 0), while that fraction was reduced two-fold in unmanipulated age-matched females (less than 40%), it can be concluded that the male to female transplants significantly reduced the prevalence of the disease. This was not the case for any of the other transplant pairs, where insulitis prevalence actually appeared higher. Importantly, in agreement with the data in Fig. 3 D and G, the effect of the male to female microbial transfers was reversed upon treatment with flutamide and blocking the AR, thereby confirming that the observed effect was indeed caused by the fluctuation in testosterone levels observed in the previous figures.
Much to the same purpose as in panel B, the researchers this time assessed anti-insulin antibody titer in NOD mice in between the different transplant groups. As evident from the strikingly lower height of the bar, autoantibodies against insulin are almost nonexistent in the females receiving male microbiota. This effect was once again abrogated by the use of flutamide, proving that it is due to the change in testosterone levels. None of the other transplant pairs had significantly lower autoantibody titer than that quantified in the control unmanipulated females (females receiving female transplants actually had significantly higher Ab titers).
Finally, in panel D, the researchers also assessed the ability of T cells from female transplant recipients to convey T1D to T1D-resistant NOD.SCID mice. The latency of the disease was significantly greater in the experimental condition using T cells from male to female transplant recipients in comparison to from unmanipulated females (13 weeks vs. 6 weeks post transfer), which could be due to either less efficient autoreactive T cells or lack thereof. Either way, the effect was again proven to be due to the conveyed increase in testosterone levels as it was annulled upon AR antagonism with flutamide.
Together, these results prove in 3 different ways that microbial transfers from mature male mice to female weanlings can significantly reduce the incidence of T1D and autoimmune disease in general.
Genomics Page
Biology Home Page
© Copyright 2014 Department of Biology, Davidson College, Davidson, NC 28035