|
*This page was produced as part of an assignment for an undergraduate course at Davidson College* GM Crops: A Farmer's Dream? |
|
Conferring Resistance Ramifications |
|
|
Biotechnology and Genetic Modification: Current Research It is important to recognize that though there are theoretical possibilities for the applications of GM technology, there are no commercially available strains and thus no existing data from large-scale field trials (Stuiver and Custers, 2001). Below you will find examples of several current research efforts into the production of disease resistance through biotechnology, these examples include:
So far, transgenic modification has been the area of conferring disease resistance through biotechnology that scientists have been putting the most energy into. Transgenic modification is, quite simply, taking the genetic material of one organism that produces a favorable trait and inserting it into another so that organism then would produce that trait. Some researchers have likened transgenic modification to taking advantage of the Earth's biodiversity (Paoletti and Pimentel, 1996). It is a fact that 90% of the human food supply is harvested from a scant 15 crop species and 8 livestock species, out of roughly 25 million species on the Earth (Paoletti and Pimentel, 1996). This cornucopia of speciation has yielded doubtless millions of genes and subsequently traits favorably selected for by evolution in these different organisms, which through science, can be applied to controlling disease in our precious crops through GM (Paoletti and Pimentel, 1996). Sometimes the referred to traits for transgenic modification can come from the most unlikely of sources and in the case of the work described by Gura, the traits came from insects (Gura, 2001). Antimicrobial peptides are a natural part of insect physiology, enabling them to be quite resilient to most forms of disease that plague crops (Gura, 2001). The genes that are responsible for encoding these antimicrobial peptides were isolated, cloned, and then shuttled into a cultivar of potatoes using Agrobacterium Tumeficans that were then exposed to late blight (Gura, 2001). Modified potatoes confirmed an expected near immunity to the late blight after modification (Gura, 2001).
A moth, one of the insects that is known to have remarkable levels of antimicrobial peptides (Gura, 2001). *Image Permission Pending* (Savela, 2003) Transgenic modification sometimes occurs by transferring traits between similar crop species. This is the case with the research done by Song and colleagues with the RB locus and potato late blight (Song et al., 2003). Researchers here observed that R genes (disease resistance genes) were naturally present in breeds of S. demissum (a species of potato) that conferred resistance to specific strains of late blight (Song et al., 2003). Furthermore, they found that there was a separate wild potato species called Solanum bulbocastanum that possessed broader resistance to all forms of late blight (Song et al., 2003). They created somatic hybrid clones through plant culturing of Solanum bulbocastanum and crop potatoes and then traced the source of resistance through targeted genetic techniques to the RB locus of genes (Song et al., 2003). From the locus, they created a bacterial artificial chromosome that can be inserted into crop species of potatoes that conferred broad based resistance to all forms of late blight (Song et al., 2003).
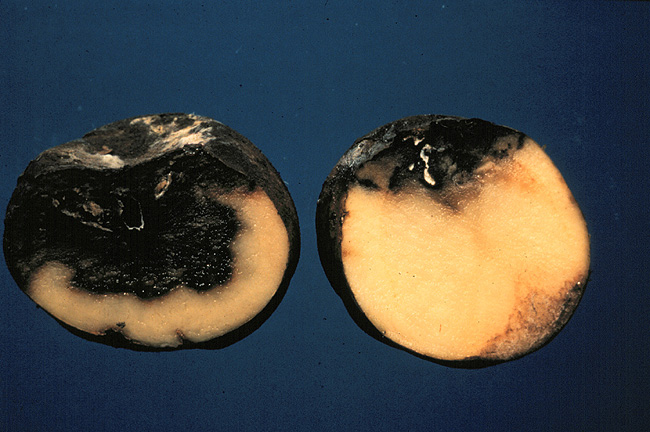
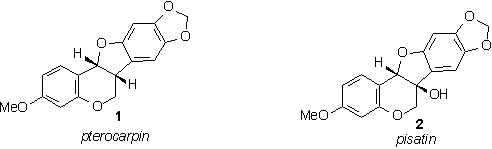
Crop potatoes infected with a blight. (Plant Pathology, 2003) *Permission Pending* The plant immune system is a very complex one indeed. One component of the immune system of a crop plant is an array of antimicrobial compounds produced as secondary products of the plant's normal metabolic processes. The class of compounds that I am referring to are often deemed phytoalexins by scientists (Dixon, 2001). These are examples of phytoalexins. (Morrow, 2003) Scientists have thoroughly diagrammed the pathway by which these products are produced and have identified a number of rate limiting steps (Dixon, 2001). It has been suggested and is being investigated that manipulation of certain rate limiting enzymes of the pathway can lead to induced overproduction of these immune products and thus greater resistance to disease for crops (Dixon, 2001). When I say rate limiting enzyme, I am referring to altering the expression of a gene that controls an enzyme that, by its operation, controls the speed of the entire pathway. It could also be possible for researchers to use transgenic modification and incorporate new classes of phytoalexins into a plant's defensive repertoire, making use of what works better in resisting forms of disease in other plants (Dixon, 2001). A more recent field exercise of this sort of modification was using genetic modification to engineer white rot resistance in sunflowers (Burke and Rieseburg, 2003). The researchers were successfully able to engineer this resistance by causing their metabolic pathway to produce more oxalate oxidase (Burke and Rieseburg, 2003). In addition to phytoalexins, the plant hypersensitive response and associated immune pathways are part of a larger network of plant disease resistance. The hypersensitive response is an effort by the plant to accomplish a quick eradication of an invading pathogen through both "damage control" and biotic assault (Stuiver and Custers, 2001). Among the many things that happen in the hypersensitive response is bombardment of the pathogen by biotic compounds produced by one of several defense pathways and reinforcement of infected cell walls with deposits of extra callose (Stuiver and Custers, 2001). Defensive products produced by these pathways are able to be manipulated in much the same way as the earlier mentioned secondary metabolic products (Stuiver and Custers, 2001). Another more intriguing method of defense pathway manipulation to induce disease resistance in a crop is "plant vaccination." There have been several researched examples of this procedure. It works very similarly to vaccination in humans, experimenters insert a benign or disabled component of a pathogen and the resultant immune response is enough to protect against all forms and reoccurrences of that disease (Waterhouse, 2001). One research effort in this topic was one of the earliest known advances in the field of genetic modification and involved engineering resistance of tobacco to the tobacco mosaic virus (Gasser and Fraley, 1989). The researchers shuttled a gene that is responsible for encoding a benign version of the tobacco mosaic virus coat protein into the cell and observed that the tobacco was completely resistant thereafter to tobacco mosaic virus (Gasser and Fraley, 1989). The mechanism by which this occurs is not completely understood today but we do know that it probably has to do with plant post-transcriptional gene silencing or PTGS (Waterhouse, 2001). PTGS is a complex system by which a plant detects viral activity and is able to silence certain pieces of DNA before they are activated and thus protect against the methodology of a virus by silencing foreign viral DNA (Waterhouse, 2001).
A diagram of the tobacco mosaic virus (Pink Monkey, 2003). *Image Permission Pending* A newer and more unorthodox method of conferring disease resistance in crop plants is by knocking out, or turning off, certain genes that make the plant more susceptible to a certain type of pathogen. An example of a gene such as this has been identified as PMR6, which encodes an enzyme that breaks down pectin (Eckardt, 2002). Why is this significant? Well, pectin is a component of plant cell walls that must remain intact during infection by a pathogen such as a fungus (Eckardt, 2002). When a fungus infects a plant cell, it attempts to extend its spores across the boundaries of an individual cell so that it can reproduce within the next cell (Eckardt, 2002). The enzyme encoded for by PMR6 breaks down a structural component of the cell wall and thus makes it weaker (Eckardt, 2002). No one understands really how a gene like this functions or why a plant would need an enzyme such as this but it was confirmed that by turning it off, the plant had a higher resistance to fungus infection (Eckardt, 2002). Another use of knocking out genes to confer disease resistance is seen in a procedure that I refer to as "damage control bypass." Nishimura found that Arabidopsis is one of several kinds of plants that, upon infection, deposit callose and reinforce infected cell walls as part of the hypersensitive response (Nishimura, 2003). Oddly enough, he also isolated a mutant variant of Arabidopsis called pmr4 that does not deposit callose upon infection by powdery mildew and yet is more resistant to the disease (Nishimura, 2003). Additionally, Nishimura found that when the salicylic acid pathway was blocked (a defense pathway) the susceptibility to powdery mildew returned for these pmr4 mutants (Nishimura, 2003). He concluded that knocking out the PMR4 gene has caused the Arabidopsis to bypass normal procedure and allocate more energy to defense rather than "damage control" (Nishimura, 2003). In other words, the plant is spreading its energy too thin naturally to adequately fight infection by depositing callose rather than trying to destroy the invading pathogen (Nishimura, 2003). Knocking out the gene causes the policy of containment to morph into a policy of eradication on the part of the plant. This change subsequently boosts the plant's defense pathways and with the now available resources the plant is able to get rid of the pathogen (Nishimura, 2003).
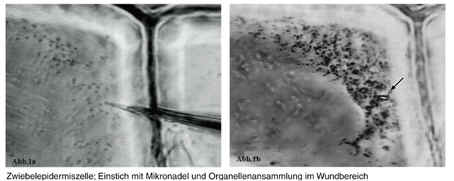
This image shows callose being deposited as part of a response to being punctured with a needle at the plant cell wall. (Lang et al., 2003). *Image permission pending*
Questions or comments can be directed to matalbert@davidson.edu |