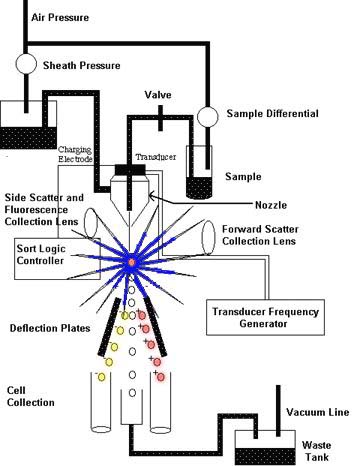

This image was obtained from a web site that is no longer available <www9uchu.edu/grad/immuno/pizzo/how.htm>
Figure #3: The FACS allows cells (lymphocytes) to be separated and isolated through the use of tagged antibodies or anti-immunoglobulins. This cartoon representation shows the major hardware of FACS, its layout, and the basic mechanisms. The sample mixture at the top of the figure is funneled through a nozzlé which isolates cells and charges them. Cells pass from the top of the figure towards the bottom. At the base of the FACS, there are 2 variably charged deflection plates and cell collectors (Lefrancois, Olson, and Pizzo; http://www9uchu.edu/grad/immuno/pizzo/how.htm).
Sample Diluting System: in order to carry out FACS, the individual cells in a resting lymphocyte pool must be isolated. This is accomplished by suspending the cells in a fluid (i.e., saline). This suspension of cells is then forced through a fine, high-pressure nozzle or fluidic diluting system which distributes the cells into a single-file line or flow cell (Kidd and Nicholson 229).
Light Source: light is an integral part of the FACS technique. Light striking each cell is scattered. By using electronic devices that measure scattered light and fluorescence, different types of cells and their sizes can be identified. In older FACS devices, the preferred light source was a mercury arc lamp. The more recent development of lasers has made the mercury arc lamp somewhat obsolete. Laser light has several advantages: it has a low amount of divergence, a high degree of intensity, and it can be adjusted to provide a variety of very specific wavelengths of light (Kidd and Nicholson 229).
Data Collecting Devices: FACSs have two types of data collecting hardware: light scatter sensors and photomultiplier tubes (PMTs). The light scatter sensors measure the light that is scattered by each cell from two different angles. The forward angle light scatter sensor (FALS) gathers light scattered in the forward direction. This type of scattered light gives a clue as to a cell's size. Right angle, orthogonal, or side light scatter (SS) sensors gather light scattered at 90° from the original direction of the light source. This light reveals cell granularity, refractiveness, and the presence of intracellular structures that reflect light (Darzynkiewicz, et al. 335). Scatter sensors are useful in distinguishing cells based on the cells' different structures. Neutrohphiles, for example, display more SS than lymphocytes (Kidd and Nicholson 229). In addition, different cell lineages or cells at different stages of development (i.e., pre B cells versus plasma B cells) can be distinguished based on their forward and side scatters (Kantor, Merrill, and Hillson 13.1). Finally, PMTs detect fluorescent emissions from the fluorescent dyes on antibodies bound to cells or from auto-fluorescence of the cells.
Integration Computer: this part of the FACS hardware is essential for collecting information from the data collecting devices and integrating it. The integrated information will be used in the formation of an electric field that will select out cells that meet certain scatter and fluorescent criteria.
Charged Plates: to collect selected cells, the cells are passed through an electric field generated by oppositely-charged plates. By changing the direction of the electric field between the plates, selected cells can be directed into precise collection areas.
References
Darzynkiewicz, Zbigniew; Xun Li; Jianping Gong; Traganos, Frank. "Methods for Analysis of
Apoptosis by Flow Cytometry." Manual of Clinical Laboratory Immunology, 5th edn. Ed. Noel R. Rose, Everly Conway deMacario, James D. Folds, H. Clifford Lane, Robert M. Nakamura. Washington, D.C.: American Society for Microbiology, 1997. 334-343.
Blue, Julia; French, T.W. "Hematology Atlas." 3 March 1997. http://drylab1.vet.cornell.edu/clinpathmodules/heme1/lymph.htm (06 October 1997).
Janeway, Charles A.; Travers, Paul. Immunobiology, 3rd Edn. New York: Current Biology Limited/Garland Publishing, Inc. 1997.
Kantor, Aaron B.; Merrill, Cynthia E.; Hillson, Jan L. "Construction of cDNA from Single Unstimulated Mouse B Lymphocytes: Method and Application to the Study of Expressed Antibody Repertoires in FACS-Sorted Murine B Cell Subsets." Immunochemistry and Molecular Immunology, vol. 1, 5th edn. Ed. Leonore A. Herzenberg, Donald M. Weir, Leonard A. Herzenberg, and Caroline Blackwell. Oxford: Blackwell Scientific Publications, 1996. 13.1-13.6.
Kidd, Pamela G.; Nicholson, Janet K.A. "Immunophenotyping by Flow Cytometry." Manual of Clinical Laboratory Immunology, 5th edn. Ed. Noel R. Rose, Everly Conway de Macario, James D. Folds, H. Clifford Lane, Robert M. Nakamura. Washington, D.C.: American Society for Microbiology, 1997. 229-244.
Lefrancois, Leo; Olson, Sarah; Pizzo, Gene. "Flow Cytometry: How Cytometry Works." n.d.. http://www9.uchu.edu/grad/immuno/pizzo/how.htm (06 October 1997).
Stadler, Beda M. "Flow Cytometry Laboratory." 27 December 1996. http://ubeclu.unibe.ch/insel/cytindex.htm (08 October 1997).
"What Is Flow Cytometry?" 07 October 1997. http://www4.umdnj.edu/cytoweb/intro.htm (07 October 1997).
© Copyright 2000 Department of Biology, Davidson
College, Davidson, NC 28036
Send comments, questions, and suggestions to: macampbell@davidson.edu