This
web page was produced as an assignment for an undergraduate course at Davidson College.
Back to my Homepage
Necrotizing Fasciitis
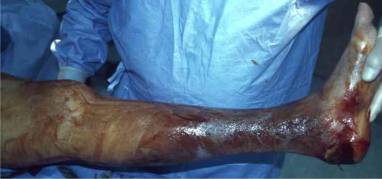
Fig 1: This is a presurgical
picture of the leg of a patient with severe
necrotizing fasciitis of the
lower leg. This picture was
part of an online
essay by Katrina Tram Duong, and was
reproduced with permission.
Necrotizing
Fasciitis(NF), commonly referred to as “flesh-eating bacteria,” is a rare yet
debilitating disease involving a rapidly progressing bacterial infection(For
particularly graphic pictures of what NF can do, visit the National Necrotizing Fasciitis
Foundation, or the Armed Forces
Infections Diseases Society). No
single species of bacterium is responsible for NF, in fact, it usually consists
of a combination of aerobic and anaerobic flora. The most common bacteria involved in NF is group A streptococcus,
Streptococcus pyogenes, the
bacterium responsible for common strep throat.
Other bacteria frequently found in NF are Staphylococcus aureus, Escheria coli, Clostridium,
Peptostreptococcus, Enterobacteriaceae, coliforms, Proteus, Pseudomonas,
Klebsiella, Bacteroides Fragilis, and Vibrio vulnificus. This varied flora makes treatment more
difficult.
It
appears that NF starts as a small local infection by one or more of these
bacteria, most frequently Streptococcus pyogenes, and/or Staphylococcus
aureus. This local infection causes
local hypoxia, under which conditions these facultative aerobes are still able
to proliferate, while leukocytes are unable to function. The aerobic respiration of these bacteria
produces a variety of gasses, which build up in tissue, causing what is
referred to as “gas gangrene.” The
rapid spread and necrotic activity of NF is enhanced by bacterial toxins, but
appears to be largely due to the overwhelming release of cytokines, much like a
localized version of Toxic Shock Syndrome(TSS). In fact, some researchers have recently identified some loci
responsible for genetic predisposition, and protection from both NF and
TSS. In 2000, a group determined that
“Severe invasive cases suffering from toxic shock and/or necrotizing fasciitis
had significantly higher frequencies of IL-2, IL-6, and TNF-alpha producing
cells in their circulation as compared to non-severe invasive
cases.”(Norrby-Teglund, et al., 2000)
Two years later, the same group(plus some) determined that the Specific
human leukocyte antigen class II locus, which is involved in the leukocyte
antigen recognition/cytokine release pathway, was at least in part responsible
for a genetic predisposition/protection for TSS/NF. Of the 11 major haplotypes they were able to identify in the
healthy population, they apparently found one haplotype that confers
protection, and one that confers predisposition to NF and/or TSS.(Kotb, et
al., 2002)
Many
other researchers have studied the mode of virulence of these bacteria, particularly
Streptococcus pyogenes. The
superantigen streptococcal pyrogenic exotoxin A(SPEA) appears to play some role
in S.pyogenes virulence, with increased levels being associated with
decreased survival in a mouse model.
However, this cannot be the primary mode of virulence, as passive
immunization against SPEA failed to exhibit protection.(Sriskandan, et al.,
1996) S.pyogenes has a number of
surface proteins that allow it to cross epithelial barriers, including M
proteins, which bind complement control factors, an hyaluronic capsule, a
cysteine protease, and fibronectin binding proteins. One group determined that “the group A streptococcal hyaluronic
capsule, and M protein, but not the cysteine protease are critical for the
development of tissue necrosis, secondary bacteremia, and lethal infection in a
murine model of human necrotizing fasciitis.(Cameron, et al., 1998) Another group demonstrated quite
dramatically that the hyaluronic capsule operates by binding CD44 in human
epithelia. “Studies of bacterial
translocation in two models of human skin[pharyngeal and skin] indicated that
cell signaling triggered by interaction of the GAS capsule with CD44 opened
intercellular junctions and promoted tissue penetration by GAS through a
paracellular route.”(Cywes, Wessels, 2001)
For
a positive patient outcome, treatment must be rapid, and aggressive. This is difficult to achieve since diagnosis
is often delayed. Patients typically
present with what appears to be a mild to moderate infection. However, within a period of days, or even
hours, NF can traverse the length of a limb, or even become systemic(at which
time it becomes TSS). Treatment
typically consists of extensive surgical debridement, along with multiple broad
range antibiotics. Penicillin G or
Clindamycin are usually given to eradicate the aerobes, while metronidazole or
a third generation cephalosporin are given concurrently to eliminate any
anaerobes. More recently, Penicillin
use has decreased, as resistance becomes more common. Penicillin works by interfering with cell wall synthesis,
Clindamycin may block t-RNA release from ribosomes, halting protein
synthesis. Metronidazole inhibits
bacterial DNA synthesis, and third generation cehpalosporins such as Rocephin
bind penicillin binding proteins.
Works
Cited
Janeway CA, Travers P, Walport M, Shlomchik M. Immunobiology: The Immune System in Health and Disease. 5th Edition. New York: Garland Publishing.
http://www.emedicine.com/emerg/topic332.htm
http://woundhealer.com/WndWebPlain/necrotizing_fasciitis.htm
http://www.hc-sc.gc.ca/pphb-dgspsp/publicat/info/necro_e.html
Ashbaugh C, Carey V, Warren H, Wessels M. 1998. Molecular analysis of the role of group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft tissue infection. J Clin Invers. 102(3):550-560.
Cywes C, Wessels M. 2001. Group A Streptococcus tissue invasion by CD44-mediated cell signaling. Nat. 414:648-51.
Kotb M, Norrby-Teglund A, McGreer A, El-Sherbini H, Dorak M, Khurshid A, Green K, Peeples J, Wade J, Thomson G, Schwartz B, Low D. An immunogenic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nat Med. 8(12):1398-1404.
Norrby-Teglund A, Chateller S, Low DE, McGreer A, Green K, Kotb M. 2000. Host variation in cytokine responses to superantigens determines the severity of invasive group A streptococcal infection. Eur J Immunol. 30(11):3247-55.
Sriskandan S, Moyes D, Buttery LK, Krausz T, Evans TJ, Polak J, Cohen J. 1996. Streptococcal pyrogenic exotoxin A release, distribution, and role in murine model of fasciitis and multiorgan failure due to Streptococcus pyogenes. J Infect Dis. 173(6):1399-407.
Send comments, questions and suggestions to edhaas@davidson.edu
Many thanks to Dr. A. Malcolm Campbell for his guidance in this endeavor as well as others. 1048266630-023226-915