This web page is created for an undergraduate class at Davidson College
Tapasin plays a role in a process that allows cells to present MHC class I molecules bound to peptide on their cell surface. MHCI molecules are loaded with peptides from within the cell. For example, when a virus infects a cell and takes over its cellular machinery to make viral proteins, portions of these viral peptides can be presented on the cell surface in an MHCI molecule. Cytotoxic T-cells recognize a particular MHCI:peptide complex on a cell can lead to apoptosis of the virally infected cell. The process by which these viral peptides are processed and presented involves many proteins. I will briefly summarize the steps required in this process and then spend the rest of the page exploring tapasin.
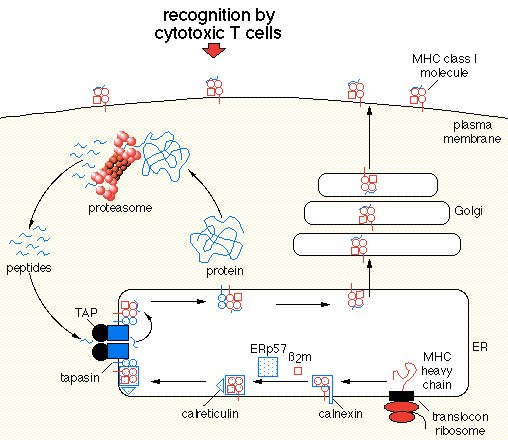
Because MHCI presents peptides from within the cell, it utilizeds the proteasome to chop up cytosolic proteins. These newly formed peptides are of the correct size to be loaded by an MHCI molecule (~8-10 amino acids). They enter the ER via the TAP complex. At this point they will be loaded into an MHCI. How does an MHCI get built and to the TAP complex? MHCI alpha chain is carried by calnexin until it binds to its B2-microglobulin chain. At this point MHCI is contructed but needs to move to TAP. Erp57, tapasin, and calreticulin move MHCI to the TAP complex. It is tapasin that forms a binding bridge between MHCI and TAP. Once in close proximity to TAP, MHCI can be loaded. Optimal loading often required peptide editing which is performed by tapasin. Once a stable MHCI:peptide complex is formed, it leaves the ER and is expressed on the cell surface. CD8+ cells recognize MHCI. If the peptide presented on an MHCI is a non-self peptide that a particular CD8+ cell's TCR recognizes, then the cell will be killed. We thus see that tapasin plays in important role in creating stable MHCI:peptide complexes that allow the immune system to identify cells that are infected from within (viruses and/or mycobacteria, etc). Check out these figures for a viualization of the above summary.

Figure1: An overview of protein degredation by a proteasome, MHCI formation and transportation to TAP, MHCI peptide loading by TAP, MHCI:peptide expression on the cell surface, and finally recognition of an MHCI:peptide complex of a cytotoxic T-cell. Image courtesy of Johann Wolfgang Goethe http://www.biochem.uni-frankfurt.de/tampe/index.html
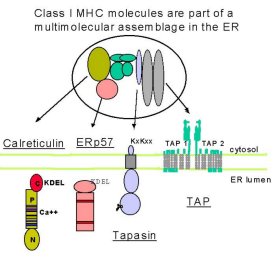
Figure 2: This figure allows us to zoom in on tapasin's role in transporting the complex to TAP. The calreticulin:Erp57:tapasin:MHCI:TAP complex. This figures shows different domains of each of the proteins involved in this pathway in which MHCI is transported to TAP in the ER membrane where it will be loaded with peptides. Image provided by http://www.som.soton.ac.uk/research/cancersciences/members/the/the.htm by Michalak et. al.
Once the alpha chian of MHCI has bound the beta-2 microglobulin, it is released from the chaperone protein calnexin. A complex of proteins next interact with this mature MHCI. These proteins include calretiuclin, Erp57, and tapasin. Tapasin serves as a 'bridge' protein for uniting this complex with the transmembrane peptide delivery protein complex, TAP. Sometimes up to four MHC:tapasin complexes bind to a single TAP complex. Tan et. al. (2002) investigated the importance of tapasin in MHCI:peptide presentation. It was previously believed that a soluble version of tapasin would lead to effective MHCI:peptide presentation, even if tapasin lost its ability to bind TAP. The group utilized a cell line that was tapasin deficient and introduced three versions of tapasin. Type 1 recieved a transmembrane point mutation (L410=>F), Type 2 recieved mouse tapasin, Type 3 recieved a mouse/human hybrid version of tapasin, and Type 4 recieved wildtype human tapasin. Apparently when MHCI peptide loading is suboptimal, MHCI molecules have a reduced half-life (lack of stability). This was the case in all Types 1-3. Only wildtype human tapasin led to optimal MHCI peptide loading and antigen recognition. Furthermore, only the wildtype human tapasin effectively led to calreticulin:MHCI:Erp57:TAP complex formation.
On this issue of TAP stability, Garbi et. al. (2003) have shown that mice who lack tapasin have a 100-fold reduction in TAP expression. They were able to activate tapsin negative cells and surprisingly these cells presented an increased amount of MHCI molecules. They conclude that despite the low level of TAP it is the presence of a large number of peptides produced by the proteasome (this was determined through the use of a protein that blocks the action of the proteasome, and this led to less MHCI presentation) that allows a relatively low number of TAP complexes to load a large number of MHCI molecules. However, these tapasin-negative cells produced MHCI:peptide complexes that were far less stable than in wildtype cells. Therefore we see that tapasin plays a role in stabilizing TAP and in optimal loading of MHCI.
The way in which tapasin leads to stable MHCI:peptide complexes is by performing 'peptide editing' (Brocke et. al. 2002). It turns out that HLA-DM and tapasin have similar (if not identicle) roles in peptide editing. Basically peptide editing means that tapasin and HLA-DM are able to remove peptides from MHCI that do not lead to a stable complex. Once the peptides are removed, another peptide can be loaded and the whole complex rechecked for stability.
Another role of tapasin is to upregulate TAP1 and TAP2. Bangia et. al. (1999) show that the C-terminal region of tapasin is sufficient to upregulate TAP as it is this end that binds TAP. They discovered that it is the final 50 amino acids that lead to a strong bond to TAP.
Reserachers are discovering more specifically the function of tapasin. Park and Ahn (2003) discuss tapasin's role in loading high affinity peptides into HLA-G. HLA-G is an MHCIb molecule which is different from MHCIa molecules because it binds a more restricted gruop of peptides. When tapasin isn't present HLA-G molecules are loaded with low-affinity peptides and thus recycled endlessly between the ER and the cis-Golgi. The hypothesis for this low affinity binding is that MHC's that bind nothing undergo ER degredation and I suppose bouncing back and forth between ER and Golgi waiting for a better peptide to be loaded is better than being degraded. When tapasin is added to the ER HLA-G molecules bind high affinity peptides and are then expressed on the cell surface. We thus see how newer research is telling us more specifically what it means for tapasin to lead to 'more stable' binding.
Ortman et. al (1997) studied the structure of tapasin and found it to be a type I transmembrane glycoprotein. It is encoded by a MHC-linked gene. Herberg et. al. (1998) mapped tapasin to within 500kbp of the TAP1/2 genes. They conclude that this points to similar regulation of these genes. It can be furthur hypothesized that TAP genes need to be inherited along with particular tapasin genes because of the importance placed on thier ability to bind. It seems reasonable that particular tapasin alleles are better able to 'bridge' with certain TAP alleles.
There are no available Chime images available for tapasin. I am pretty surprised by this. Perhaps it is a particularly difficult protein to crystalize because it is very well studied. Chen et. al. (2002) determined that tapasin has two core domains linked by a flexible region at amino acids ~85-93.. The primary structure is beta sheets. Perhaps we can infer that this 'hinge' region acts as a bridge between MHC and TAP, or perhaps it allows for loading of peptides.
I will include here a chime image of an MHCI bound to peptide.
Figure 3: Tap-B-Associated Rat Mhc Class I Molecule. This shows high affinity binding of a peptide by an MHCI molecule. Remember that tapasin plays a role in peptide editing to load such a high affinity peptide. I recomend viewing this image by first going to Color and choosing Chain. The blue is the heavy alpha chain, the green is the B2-microglobulin, and the yellow is the peptide bound by MHCI. Image provided by PDB. (Rudolph et. al. 2002)
We have seen from Tan et. al. (2002) that tapasin is required for proper loading of MHCI. We have also seen that tapasin upregulates TAP1/2. Therefore loss of tapasin is associated with would seem to lead to similar phenotypes as loss of TAP. This is in fact the case as demonstrated by Yabe et. al. (2002). The disorder known as bare lymphocyte syndrome (BLS). Previously it was only associated with loss of TAP. This study identified a patient with BLS symptoms (reduced MHCI:peptide on the cell surface) but completely normal TAP. They determined that the patients tapasin had undergone a 7.5 kb deletion and thus no tapasin was found in the cell. It is worth noting that the MHCI reduction in this patients BLS was not as pronounced as in patients with BLS caused by effected TAP.
Because viral peptides are presented on MHCI molecules that will signal for cell death, it would make sense for viruses to evolve mechanisms for combating MHCI:peptide expression. Of course we must remember that another way to kill your host cell is to keep it from presenting MHCI at all. One interesting viral response to this system is discussed by Lybarger et. al. (2003). They study gamma(2)-Herpesvirus 68 and its ability to disrupt loading and presentation of MHCI:peptide complex. This virus makes a protein called mK3 which interacts with TAP/tapasin. Essentially this protein binds the TAP/tapasin complex and somehow leads to destruction of MHCI.
Garbi N, Tiwari N, Momburg F, Hammerling GJ. A major role for tapasin as a
stabilizer of the TAP peptide transporter and consequences for MHC class I expression.
Eur J Immunol 2003 Jan;33(1):264-73
Momburg F, Tan P. Tapasin-the keystone of the loading complex optimizing peptide binding by MHC class I molecules in the endoplasmic reticulum. Mol Immunol 2002 Oct;39(3-4):217-33 .
Brocke P, Garbi N, Momburg F, Hammerling GJ. HLA-DM, HLA-DO and tapasin: functional similarities and differences. Curr Opin Immunol 2002 Feb;14(1):22-9
Bangia N, Lehner PJ, Hughes EA, Surman M, Cresswell P. The N-terminal region of tapasin is required to stabilize the MHC class I loading complex. Eur J Immunol 1999 Jun;29(6):1858-70
Park B, Ahn K.J An essential function of Tapasin in quality control of HLA-G molecules. Biol Chem 2003 Feb 11
Ortmann, B.; Copeman, J.; Lehner, P. J.; Sadasivan, B.; Herberg, J. A.; Grandea, A. G.; Riddell, S. R.; Tampe, R.; Spies, T.; Trowsdale, J.; Cresswell, P. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science 277: 1306-1309, 1997.
Yabe T, Kawamura S, Sato M, Kashiwase K, Tanaka H, Ishikawa Y, Asao Y, Oyama J, Tsuruta K, Tokunaga K, Tadokoro K, Juji T. A subject with a novel type I bare lymphocyte syndrome has tapasin deficiency due to deletion of 4 exons by Alu-mediated recombination. Blood 2002 Aug 15;100(4):1496-8
Marek Michalak, University of Alberta, Canada; Jim McCluskey, University of
Melbourne, Australia; Paul Eggleton, University of Oxford, UK. http://www.som.soton.ac.uk/research/cancersciences/members/the/the.htm
March 20, 2003.
Herberg JA, Sgouros J, Jones T, Copeman J, Humphray SJ, Sheer D, Cresswell P, Beck S, Trowsdale.Genomic analysis of the Tapasin gene, located close to the TAP loci in the MHC. J.Eur J Immunol 1998 Feb;28(2):459-67
Lybarger L, Wang X, Harris MR, Virgin HW. Virus subversion of the MHC class I peptide-loading complex.Immunity 2003 Jan;18(1):121-30.
Johann Wolfgang Goethe-Universität Frankfurt am Main Institute of Biochemistry, Biocenter http:://www.biochem.uni-frankfurt.de/tampe/index.html
Rudolph MG, Stevens J, Speir JA, Trowsdale J, Butcher GW, Joly E, Wilson IA.Crystal structures of two rat MHC class Ia (RT1-A) molecules that are associated differentially with peptide transporter alleles TAP-A and TAP-B. J Mol Biol 2002 Dec 13;324(5):975-90.
Chen M, Stafford WF, Diedrich G, Khan A, Bouvier M. A characterization of the
lumenal region of human tapasin reveals the presence of two structural domains.
Biochemistry 2002 Dec 10;41(49):14539-45.
Email Kevin James with comments or questions