What are the symptoms of Goodpasture's
Syndrome?
Normal Renal Anatomy
Progress of Goodpasture's Syndrome
Basement
Membrane Damage
Production
of Anti-GBM Antibodies
Tissue
Damage
Treatment of Goodpasture's Syndrome
Works Cited
Back
to Home Page
What are the symptoms of Goodpasture's Syndrome?
Patients with
Goodpasture's Syndrome (referred to from here as GS) usually present with
pulmonary hemorrhage, evidenced by hemoptysis (coughing up blood), and
glomerulonephritis, evidenced by hematuria (blood in the urine) (Avella
et
al., 1999). While more than 50% of GS cases present with pulmonary
hemmorhage, some patients also present with no renal symptoms (Avella et
al., 1999; Kuzmanic et al., 1999). These patients
may be mid-way through the course of GS, as pulmonary hemorrhage may precede
renal symptoms by weeks or months (Avella et al., 1999). The
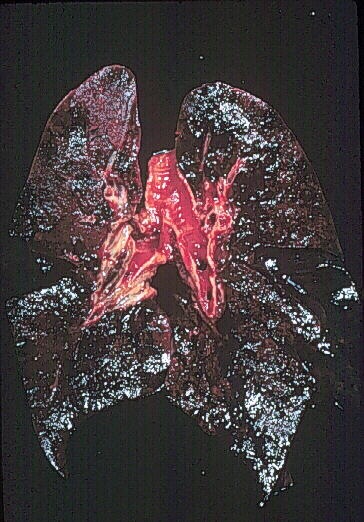
lung pathology of a patient who died of GS is shown below. Note the
extensive necrosis (black areas as opposed to the pink area in the upper
left quandrant):

Lung pathology of Goodpasture's Syndrome. Used with permission of author. Source: http://www.gamewood.net/rnet/renalpath/ch1.htm
Before we discuss the disease pathology,
it would be useful to review normal renal pathology. GS involves
the basement membranes, which form a barrier whenever cells (specifically
in organs) meet connective tissue (Hellmark et al., 1996).
The basement membrane has a stable skeleton of Type IV collagen, onto which
other basement membrane molecules, such as proteoglycans, attach (Hellmark
et
al., 1996). Spaces in the basement membrane enable filtration of large
amounts of water and small solutes, but electrostatic interactions with
proteoglycans prevent filtration of plasma proteins from the blood (Guyton
et
al., 1996). Below is a schematic image of a normal glomerulus.
Note the position of the basement membrane.
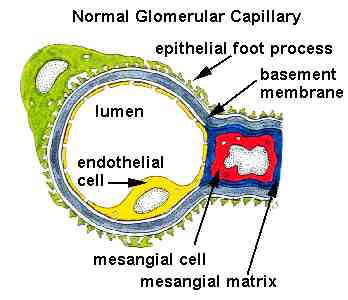
Normal Glomerular Capillary. Used with permission of author. Source: http://www.gamewood.net/rnet/renalpath/ch1.htm
Each strand of Type
IV collagen is composed of three subunits, or a
(IV) chains (Hellmark
et al., 1996). These a
chains may be grouped into six genetically distinct categories, with a1
and a3 being of particular interest when studying
GS (Hellmark et al., 1996). The basement membranes of the
glomeruli and alveoli share a unique combination of a1
and a3 Type IV collagen (Hellmark et al.,
1996).
Progress of Goodpasture's Syndrome
Basement Membrane Injury
Goodpasture's
Syndrome begins with injury to the basement membrane that exposes the Type
IV collagen backbone (Kalluri, 1999). Proposed routes of basement
membrane injury include smoking, inhalation of volatile hydrocarbons or
other toxins, renal injury, incidental glomerulnephritis or ischemia (Kalluri,
1999; Avella et al., 1999).
Production of Anti-GBM Antibodies
After exposure
of the collagen in the basement membrane, the body intiates an autoimmume
reaction to the a3 chain of the Type IV collagen.
These antibodies against a3 chain of the Type
IV collagen are also called anti-Glomerular Basement Membrane (GBM) Antibodies
or Goodpasture's antibodies. The inappropriate immune response may
be enabled by deficiency in an Fc receptor.
Fc receptors
bind to the Fc portion of immunoglobulin (Ig) molecules (Janeway et
al., 1999). The specificity of the receptor is based on recognition
of the alpha domain on the Fc molecule (Janeway et al., 1999).
FcgR-IIB is one type of Fc receptor that is
expressed on macrophages, neutrophils, eosinophils, B cells and mast cells
(Janeway et al., 1999). FcgR-IIB
expression on B cells may prevent activation of low-affinity autoreactive
cells during affinity maturation and may also prevent the development of
autoreactive memory cells in the germinal centers (Nakamura et al.,
2000). The FcgR-IIB receptor inhibits
the response of these autoreactive cells when crosslinked with the B cell
receptor (BCR) by binding to SHIP (Nakamura et al., 1999; Janeyway
et
al., 1999). The FcgR-IIB receptor
may also trigger apoptosis when it crosslinks to itself without BCR (Nakamura
et
al., 1999).
Animals deficient
for FcgR-IIB have stronger immune responses
and more inflammation in all antibody-mediated types of hypersensitivity
reactions than animals with normal expression of the receptor (Nakamura
et
al., 1999). When the FcgR-IIB mice
are immunized with Type IV collagen, they develop an autoimmune disorder
similar to GPS: pulmonary hemorrhage and nephritis consistent histologically
with the pathology of GPS (Nakamura et al., 1999). This tissue
damage did not occur systemically, supporting the idea that the pathology
was not the result of generalized inflammation (Nakamura et al.,
1999). The tissue damage associated with GPS is thought to be caused
by the anti-GBM antibodies binding to the a3
chain of the Type IV collagen in the basement membrane of the glomeruli
and alveoli (Nakamura et al., 1999). These antibodies bind
and activate effector cell responses (Nakamura et al., 1999).
Tissue Damage
The recruitment
of effector cells and the subsequent inflammatory response decreases the
blood flow to the glomerulus (Avella et al., 1999). This stimulates
formation of crescents in the glomerulus, as seen below (Kaplan, 1997).
* compare with first schematic image of a normal glomerulus,
with the open lumen.
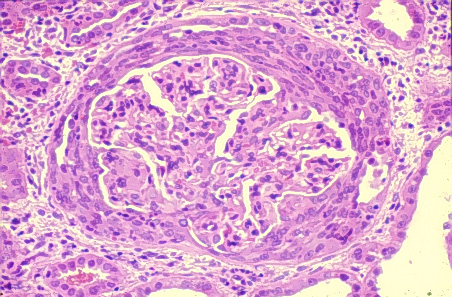
Crescent formation in glomerulonephritis associated with Goodpasture's
Syndrome. Used with permission of authors. Source:
http://www.gamewood.net/rnet/renalpath/ch1.htm
Another mechanism
for Goodpasture's syndrome is that susceptible MHC-II alleles may bind
selectively to a3 chain of the Type IV collagen,
prompting T cell recognition of these fragments (Kalluri, 1999).
Goodpasture's syndrome has been correlated with HLA DR2 and DR4 alleles
(Levy et al., 1996).
Goodpasture's
syndrome is a unique autoimmune reaction in that it is monophaic:
it occurs only once (Levy et al., 1996). Recurrence of clinical
symptoms or anti-GBM antibodies is very rare; only ten cases of recurrence
have been reported (Levy et al., 1996). This may be because
the antigen (a3 chain of the Type IV collagen)
is usually "hidden" in the intact basement membrane (Levy et al.,
1996). Since the body is not continuously exposed to the antigen,
it may recover from the initial autoimmune reaction (Levy et al.,
1996). This is not to say that most people recover from Goodpasture's
Syndrome with no medical intervention, only that those who do receive medical
support will usually not have a recurrence of the syndrome.
How is Goodpasture's Syndrome Diagnosed?
When a patient presents with the initial
indicators of Goodpasture's Syndrome (hemoptysis and hematuria), an anti-GBM
antibody titer, bronchoscopy and renal biopsy may provide a definitive
diagnosis of GS (Avella et al., 1999). The anti-GBM antibody
titer is indicated when patients have acute renal failure, pulmonary hemorrhage
and/or rising serum creatine concentrations with hematuria (Hellmark
et al., 1997). The Anti-GBM antibody titer may eliminate other
disorders, as these symptoms may be indicative of other diseases (Hellmark
et
al., 1997). A definitive diagnosis of GS will include an anti-GBM
Ab titer test of greater than 20 units (normal = 0-9 units), bloody secretions
in the bronchoscopy, and a linear distribution of IgG on the basement membrane
of the renal biopsy (Avella et al., 1999). An example of the
linear distribution of IgG on the glomerular basement membrane is shown
below:
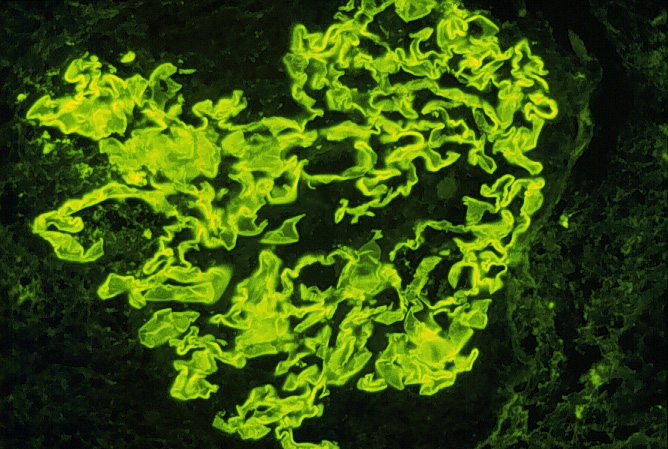
Immunoflorescence image of linear distribution of IgG on glomerular basement membrane Used with permission of author. Source: http://www.gamewood.net/rnet/renalpath/ch1.htm
Treatment of Goodpasture's Syndrome
The traditional course of treatment for GS has been pulse-dose
of steroids (methyl prednisone), followed by oral steroids and immunosuppressants
(cyclophosphamide), with initiation of plasmapheresis at the diagnosis
of GS (Hellmark et al., 1997). The immunosuppressants inhibit
synthesis of new anti-GBM antibodies, while the plasmapheresis removes
existing anti-GBM antibodies and complement factors from the blood (Harada
et
al., 1998; Kaplan, 1997). In a comparison of GS patients who
received immunosuppressants alone versus patients receiving immunosuppressants
and plasmapheresis, the dual-treatment group experienced a faster decline
in anti-GBM antibodies and had half the serum creatine level of the single
treatment group (Harada et al., 1998). The efficacy of plasmapheresis
has been disputed, though, and one clinician noted that early diagnosis
and initiation of treatment is the best indicator of positive renal outcome
(Kaplan, 1997).
Works Cited
Avella, P et al. Goodpasture's Syndrome: A Nursing Challenge. Dimensions in Critical Care Nursing. 18(2): 2-11. Mar-Apr 1999.
Guyton, A et al. Textbook of Medical Physiology. 9th ed. Philadelphia, PA: WB Saunders Company. 1996.
Harada, T et al. Therapeutic Apheresis for Renal Diseases. Therapeutic Apheresis. 2(3): 193-98. 1998.
Hellmark, T et al. Anti-GBM Antibodies in Goodpasture Syndrome; anatomy of an epitope. Nephrology, Dialysis and Transplant. 12: 646-48. 1997.
Janeway, C et al. Immunobiology: The Immune System in Health and Disease. 4th ed. New York: Garland Publishing. 1999.
Kalluri, R. Goodpasture's Syndrome. Kidney International. 55: 1120-22. 1999.
Kaplan, A. Therapeutic Plasma Exchange for the Treatment of Rapidly Progressive Glomerulonephritis. Therapeutic Apheresis. 1(3): 255-59. 1997.
Kuzmanic, D et al. Goodpasture's Syndrome with Normal Renal Function. Clinical Nephrology. 51(5): 319-20. May 1999.
Levy J et al. Recurrent Goodpasture's Disease. American Journal of Kidney Diseases. 27(4): 573-78. April 1996.
Nakamura A et al. Fc gamma Receptor IIB-deficient
Mice Develop Goodpasture's Syndrome upon Immunization with Type IV Collagen:
A Novel Murine Model for Autoimmune Glomerular Basement Membrane Disease.
Journal of Experimental Medicine. 191(5): 899-905. March
6, 2000.
Now you can return to the Molecular Biology Home Page, or Immunology Home Page.