Affinity Chromatography
Affinity chromotography, also known as bioselective adsorption, is a protein purification technique developed thirty years ago. The fundamental principal composing the method is the isolation of a particular protein by chromatography on a bioligand that has been immobilized on an inert matrix. It relies on the specificity of a protein binding site for a particular ligand. For this precise coupling to take place a number of variable conditions must be addressed first.
Sample Before any attention can be paid to the actual technique, a protein sample is needed. Once the desired protein's tissue location has been determined, cells comprising this tissue must be isolated. Once done, a crude cellular extract is performed and all endogenous substrates are removed. The sample is now ready tfor the column.
Matrix A sound matrix is an essential part of affinity chromatography. A matrix, in its use here, is a substance, usually in bead form, to which a specific ligand is covalently bound. In order for the matrix to be affective it must have certain characteristics: 1) it must be insoluble in solvents and buffers employed in the process; 2) it must be chemically and mechanically stable; 3) it must be easily coupled to a ligand or spacer arm onto which the ligand can be attached; 4) it must exhibit good flow properties and have a relativley large surface area for attachment. It is common for matrices to be made out of agarose, glass, cellulose, or a duel composition polyacrylimide based compound. (Scouten 20)
Solvents The primary buffer in affinity chromatography is the one in which the matrix resides. This buffer should not degrade the matrix in any way. The buffer should also have a negliable effect on the sample. The ideal buffer minimizes nonspecific interactions while maximizing the specific interaction between the sample and the ligand.
The other major solvent to consider in affinity chromatography is the elution buffer. The purpose of the elution buffer is to wash away unbound proteins initially and at higher concentration release the desired protein from the ligand. Salt solutions of various concentrations as well as buffers containing specifc analogs for the sample can be used. It is important that the elution buffer work quickly and to not change the function or activity of the desired protein.
Spacer arms and coupling methods Since the success of affinity chromatography resides in its ability to bind an active site to its corresponding ligand, if the protein binding region cannot join with the immobilized ligand the technique is effectively useless. Spacer arms, though not always necessary, can improve binding probability. Spacer arms distance the ligand from the matrix reducing steric hinderance which can occur when the ligand is bound directly to the bead. Spacer arms should neither chemically or structually affect the sample or the ligand. (Scouten 38)
When coupling the spacer arm or ligand to the matrix, multipoint or single point attachment may be used. Single point attachment offers high ligand flexabilty and easier ligand access to the sample's active site. Though single point coupling does provide better site recognition, it is not nearly as strong as a multipoint attachment. Multipoint coupling is stronger than single point attachment and will thus show less degradation. Unfortunately it can impede binding between the ligand and the sample.
After the ligand has been bound to the matrix it is important as a final step to block all unreacted groups of the matrix. This provides a higher degree of certainty that all binding will be between the sample and the ligand. (Deutscher 363)

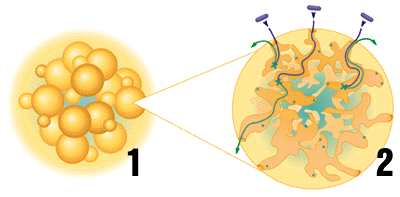
Fig1. This is a depiction of a matrix bead showing ligand and protein binding. The X's represent a ligand and the pill-shaped images represent a protein. The image comes from the Bio-Rad homepage.
Pouring the column Once the ligand has been immobilized, the column packed, and the sample prepared the sample can finally be poured. Since ligand binding is based on hydrogen bonding, site/ligand motifs and other noncovalent interaction, a certain degree of care must be used to make binding as opportune as possible. Variations in flow rate of the sample, and of the wash and elution buffers can exhibit monumental effects on the success of affinity chromatography. If the sample is poured too quickly proper binding may not take place. If, when pouring the wash, the flow rate is too high the bound protein may release as well. And finally, though the elution may be at a higher flow rate, if it exceeds the rate used to pack the original matrix the entire complex may come apart. (Deutscher 365)
Just as impotant as flow rate is the concentration and acidity of the buffers used. As mentioned above, buffers should in no way chemically or structually interfere with ligand binding. In the elution phase it is necessary for buffer to seperate the ligand from the sample, but prior to that buffers should remain inocuous because of the sensitive ligand/sample interaction.

Fig 2. This is a depiction of a column used in affinity chromatography. The image comes from the Bio-Rad hompage.
What do now After elution, a protein, specific to the ligand, has been isolated. To determine protein purity and activity SDS-PAGE or immunoelectrophoresis can be used.
Applications Affinity chromatography has a large range of protein purfying applications. For example, the control of transcription factors to activate DNA transcription makes them a extremely important to understanding gene expression. Using a a specific DNA sequence as a ligand, these proteins can be isolated via affinity chromatography. Another major group is enzymes. Enzymes can be isolated by a host a different ligands fit for bioselective adsoprtion. For example, adenosine monophosphate (AMP) can be immobilized and used to bind those proteins exhibiting an affinity for AMP, ADP, or ATP. Extra cellular and other receptor proteins can also be purified by affinity chromatography. For example, determination of whether a particular hormone has recpetor in a specific tissue group can be accomplished by using the hormone in question as the immobilized ligand. These examples are only a small sample of the vast applications of affinity chromatography. (Ngo 4-9)
Affinity chromotography is a powerful technique in that it allows protein purifiaction in a relatively short amount of time with a high yield. It simplifies the isolation process by using the preexisting ligand binding relationships already in an organism.
References
Deutscher, Murray P. Methods in Enzymology: Guide to Protein Purifcation. San Diego: Academic Press Inc., 1990.
Ngo, That T. Molecular Interactions in Bioseperations. New York: Plenum Press, 1993.
Scouten, William H. Affinity Chromatography. New York: John Wiley & Sons, 1981.
Need to get back to Dan's homepage click here