Phospholamban

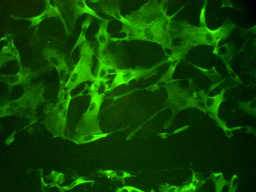
Figure 1. The expression of phospholamban in mammalian smooth muscle cells. [Source: P. Cummins. Permission of image pending.]
This page is meant to serve as an introduction to the properties of the protein phospholamban.
From this page, you will learn about the role that phospholamban plays, how it works, and where it is located in organisms. Additionally, you will learn about phospholamban's mutants and their problematic effects.
What is Phospholamban (PLB)?
PLB is a 6-kD pentameric protein with each polypeptide subunit being 52 amino acids long. The pentameric complex is a left-handed coiled-coil of five alpha-helices. In living organisms, it can be found in both its pentameric and monomeric forms (Arkin and others 1995).
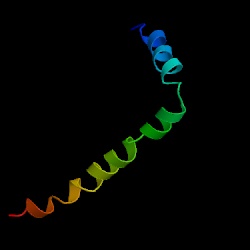
Figure 2. The structural image of PLB in monomeric form. This particular depiction is of chain A and does contain a mutation. [Source: Protein Data Bank. Permission of image pending.]
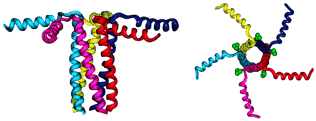
Figure 3. PLB in pentameric form. The compound on the right is PLB while the compound on the right is sarcolipin, a protein similar to PLB in that it too regulates Ca-ATPase. [Source: Minnesota Muscle Laboratory. Permission of image pending.]
PLB is an integral protein; it is composed of an N-terminal cytoplasmic domain
(30 amino acid residues) and a C-terminal membrane-spanning domain (22 amino
acid residues). The N-terminal residues are mostly hydrophilic and it has two
phosphorylation sites that are involved with the regulation of the calcium pump.
The C-terminal residues are mostly hydrophobic; they anchor the protein and
they are responsible for the selective ion conductance of Ca(2+) (Reddy and
others 1999). Therefore, PLB has two important features: the cytoplasmic phosphorylation
sites and the ion pore formed by the interaction of the 5 helical subunits.
What does PLB do?
PLB is a major substrate for the cAMP-dependent protein kinase located in the cardiac muscles, but it is also expressed in slow twitch skeletal muscle and smooth muscle cells. In the cardiac muscle, PLB indirectly controls the activation of sarcoplasmic reticulum calcium(2+)-ATPase (SERCA). It In the unphosphorylated state, PLB is an inhibitor of cardiac muscle SERCA, however when PLB is phosphorylated, there is no inhibition. This lack of inhibition results in the activation of the Ca(2+) pump, which leads to enhanced muscle relaxation rates, which in turn contributes to the inotropic response (a stronger contraction of cardiac muscle) that is elicited in the heart by beta-agonists (OMIM 2000).
How does PLB work?
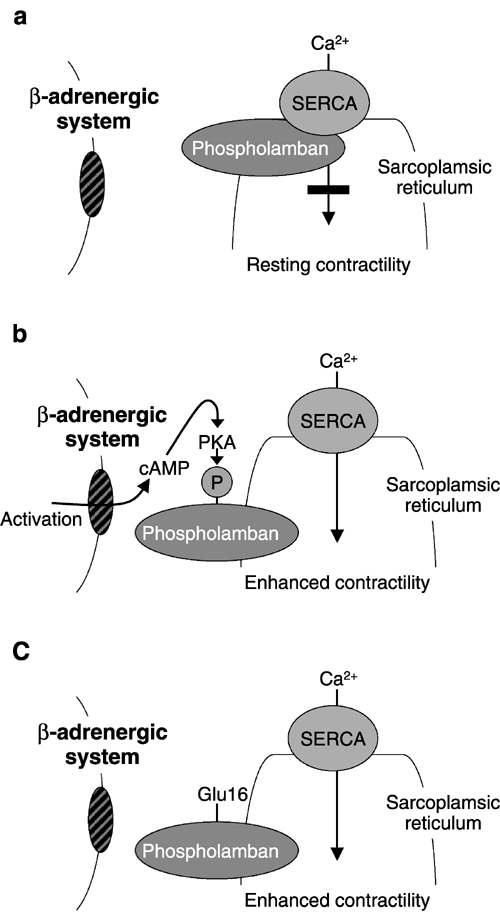
Figure 1 Contractile function of heart cells. (a) Normal heart, resting. Nonphosphorylated phospholamban binds to sarcoplasmic reticulum Ca2+-ATPase (SERCA), suppressing Ca2+ cycling, maintaining a resting level of contractility. (b) Normal heart, activated. Phosphorylation of phospholamban removes the resting SERCA suppression, enhances SERCA-mediated Ca2+ cycling, and thus contractility. (c) Gene therapy with S16E phospholamban. Gene transfer of a pseudophosphorylated form of phospholamban (S16E) results in persistent SERCA-mediated Ca2+ cycling, and thus persistent enhanced contractility. [Source: Nature Publishing Group.]
Mutations of PLB
PLB in the News
Less than a month ago (in February of 2003), Science magazine published an article by Joachim Schmitt and colleagues that revealed new evidence of the role of PLB in dilated cardiomyopathy. This heart-muscle disorder occurs when diseased muscle fibers cause the heart to enlarge and thereby weaken its ability to pump. Dilated cardiomyopathy has been shown to be inherited in some patients, and Schmitt et al. discovered that a mutation in PLB is the cause of the defect (Norton 2003).
References
Arkin TA, Rothman M, Ludlam CFC, Aimoto S, Engelman DM, Rothschild KJ, Smith SO. 1995. Structural model of the phospholamban ion channel complex in phospholipid membranes. J Mol Biol 248:824-834.
Norton A. 2003 Feb 27. Calcium Defect May Be a Cause of Heart Failure. Reuters Health. <http://www.nlm.nih.gov/medlineplus/news/fullstory_11828.html>. Accessed 2003 Mar 12.
Reddy LG, Autry JM, Jones LR, Thomas DD. 1999. Co-reconstitution of phospholamban
mutants with the Ca-ATPase reveals dependence of inhibitory function on phospholamban
structure. J Biol Chem 274(12):7649-7655.
Stokes DL. 1997. Keeping calcium in its place: Ca(2+)-ATPase and phospholamban. Curr Opinion Structural Biol [serial online] 7:550-556. <http://saturn.med.nyu.edu/~stokes/ms/ stokes-curr-opinion-caplb.pdf> Accessed 2003 Mar 9.
Online Mendelian Inheritance in Man, OMIM (TM). McKusick-Nathans Institute for Genetic Medicine, Johns Hopkins University (Baltimore, MD) and National Center for Biotechnology Information, National Library of Medicine (Bethesda, MD), 2000. <http://www.ncbi.nlm.nih.gov/omim/>
Please send your questions, comments, or concerns to Suzy Rizi