This page was created as part of an undergraduate course at Davidson College
Horseradish Peroxidase
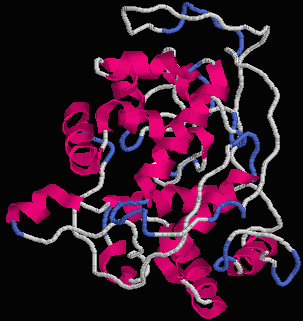
Figure 1. The chime structure of Horseradish Peroxidase. [Source: M. Pravda]
This page is intended to further your understanding of how HRPs can be used as tools in both ELISAs and Western Blots.
Read on to discover the wonderful world of HRPs!
What is Horseradish Peroxidase (HRP)?
HRP is an enzyme-label that is commonly used in conjunction with a secondary antibody to detect proteins. It is extracted from the roots of horseradish using mild affinity chromatography. It has high levels of both activity and specifity and is therefore a preferred enzymatic label in procedures such as ELISA, Western Blots (immunoblotting), and immunohistochemistry (Horseradish Peroxidase 2000).
What is an Enzyme-Linked Immunosorbent Assay (ELISA)?
The ELISA is a method to determine the concentrations (on the order of ng/mL to pg/mL) of a material in a solution. The ELISA will measure optical density and using that information, you can determine the concentration of the protein of interest. In order to do this, you can create a standard curve graph for the optical density versus the concentration of the protein; extrapolation from this curve will yield the concentration of the protein. For more information on this tool, click here.
How is HRP used in an ELISA?
HRPs are used as secondary antibodies in ELISAs. Therefore, the HRP will bind to a primary antibody, which binds to the target protein (the target protein is the protein that you wish to detect). The HRP will then bind to a substrate to yield a signal that leads to detection; HRP catalyzes a reaction with the substrate that causes the color change (Schutz and others 1997).

Figure 2. How HRP might work in an ELISA (or Western Blot). In this example, the primary antibody has been modified with phenyldiboronic acid (PDBA) in order to increase binding properties to the HRP (or AP, alkaline phosphatase). The key point to understand in this diagram is that HRP binds to the primary antibody (in red) and when the substrate binds to HRP, a signal is elicited. [Source: Versalinx. Permission of image pending].
How do you perform an ELISA using HRP?
Step 1: Coat a solid phase membrane with an antigen and a buffer that enhances binding and a blocking buffer to prevent non-specific binding of the protein
Step 2: Add the serum sample and incubate. In this step, the antibody that is specific to the antigen will bind.
Step 3: Wash away the non-specific antibodies.
Step 4: Incubate with an HRP-labeled secondary antibody. The secondary antibody will bind tightly to the primary antibody.
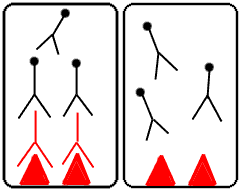
Step 5: Wash away the unbound labeled antibodies.
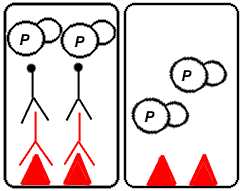
Step 6: Add a colorimetric substrate (such as o-phenylenediamine dihydrochloride). The enzyme will cleave the substrate, causing a color change in the substrate solution. The intensity of the color change is quantified using a spectrophotometer.
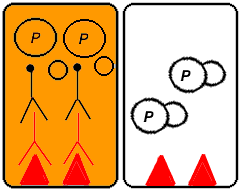
Figure 2. A series of cartoons of the ELISA procedure (Step 1 through Step 6). The images on the left depict what happens to proteins that bind the primary and HRP-conjugated secondary antibodies (ideally, the target proteins). The images on the right depict what happens to proteins that do not bind either of the antibodies (ideally, all non-target proteins). [Source: Sound Diagnostics, Inc. Permission of images in this section pending.]
Step 7: Make a standard curve based on the fact that the intensities are proportional to the amount of antibody (and therefore target protein) in the sample. Use the standard curve to determine the concentration of the desired sample.
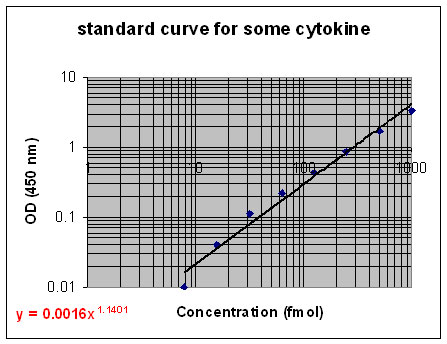
Figure 3. An example of what a standard curve for a protein (in this case a cytokine) might look like. The equation in red is the power equation that may be used to determine the concentration of the protein as a function of the optical density. [Source: O. Mesarwi.]
What is a Western Blot?
A Western Blot is a tool used by molecular biologists to determine the molecular weights of proteins and to determine the relative amount of protein in a sample. For more information on this tool, click here. In the Western Blot, HRP, in the presence of hydrogen peroxide, functions by catalyzing a reaction involving the oxidation of luminol (see Figure 4). Upon the oxidation of luminol, an iridescent blue light is produced and that light is detected by chemiluminescence. While the oxidation of luminol is not dependent on HRP, the enzyme is necessary for enhanced chemiluminescence (Ilyina and others 2000).
How do you perform a Western Blot using HRP?
Step 1: Extract protein from cell.
Step 2: Separate proteins via SDS-PAGE (or another form of gel electrophoresis).
Step 3: Transfer proteins to nitrocellulose paper (blotting).
Step 4: Block with excess protein.
Step 5: Incubate the blot with a primary antibody (to target the protein of interest)
Step 6: Wash away the unbound probes.
Step 7: Incubate the blot with a secondary antibody conjugated to HRP.
Step 8: Wash away the unbound labeled-probes.
Step 9: Develop via chemiluminescence. (A common method of developing this blot is the Pierce Chemiluminescence Method. For more information on this, click here.)
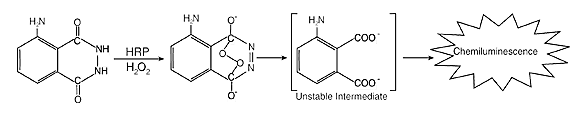
Figure 4. How the substrate is induced to create chemiluminescence via HRP in the presence of hydrogen peroxide. [Source: Roche Applied Science. Image used by the permission of Roche Diagnostics.]
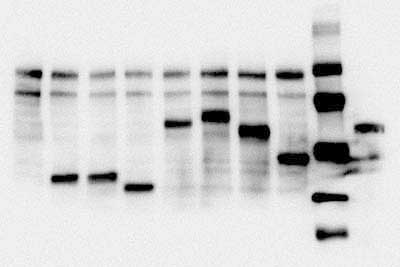
Figure 5. A typical experimental result of a Western Blot using an HRP-labeled antibody probe developed via chemiluminesce. [Source: Roche Applied Science. Image used by the permission of Roche Diagnostics.]
Why is HRP an important tool?
As an enzyme, HRP serves diverse functions, as exemplified in its use in both ELISAs and Western Blots. Where before radioactive measures were necessary to perform such tests, HRP has allowed the standardization of non-radioactive detection of nucleic acids and proteins. It is a popular tool in the world of molecular biology because the reactions that it catalyzes have high substrate specificity, both regio- and enantioselectivity, and very little (if any) bi-product formation (Cho and others 1999). Use of HRP has become common in medical research, including neuroanatomy. HRP was recently found to bind to the terminal end of neurons. Neurons will transport the HRP to the soma (or cell body). This process is an example retrograde transport; the neurons transport the HRP "backwards" from the terminal of the neuron to the cell body. Therefore, it allows us to detect what areas of the brain connect with other areas of the brain (Vesselkin and others 2001).
Please send your questions, comments, or concerns to Suzy Rizi