*This web page was produced as an assignment for an undergraduate course at Davidson College.*
Hemoglobin
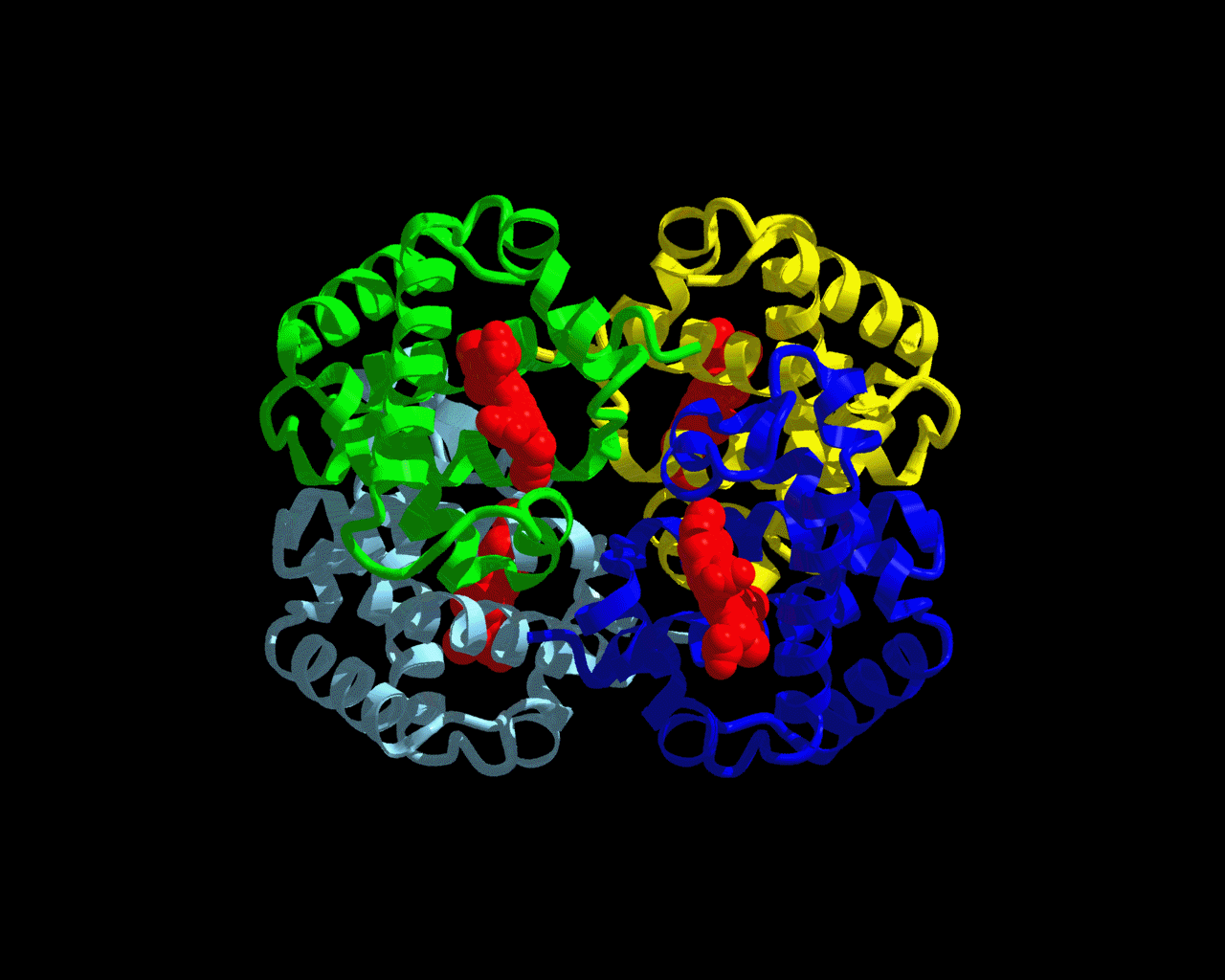
Figure 1: Hemoglobin Structure
The structure seen above is hemoglobin. The green, yellow, blue, and gray colors make up the four polypeptide subunits of hemoglobin. Each subunit has its own heme group (shown in red.) The heme group is where the oxygen binds. This picture can be accessed at http://www.psc.edu/science/Ho/Ho.html.
What is hemoglobin?
Hemoglobin is a red pigment in red blood cells that can bind with oxygen. This protein is responsible for binding oxygen in the lung and transporting the oxygen throughout the body to be used up in aerobic metabolic pathways. Hemoglobin is a protein made up of four polypeptide subunits, as seen in the picture above. Within each one of these subunits is a heme group. A heme group is an iron-containing ring structure that has the ability to bind to oxygen. Each hemoglobin protein can bind to four oxygen molecules - one oxygen molecule for each heme group.
Hemoglobin Image
Figure 2: This PDB file was obtained from http://www.rcsb.org/pdb/. Right click on structure for more options.
What helps hemoglobin maintain its quaternary structure?
1) Hydrophobic interactions - These interactions group together in the interior of the protein, away from water, causing the polypeptide to fold.
2) Van der Waals forces - These forces stabilize the close interactions between the hydrophobic residues.
3) Hydrogen bonds - Chemical bonding between positively charged hydrogens and negatively charged atoms such as fluorine, oxygen, or nitrogen.
4) Ionic bonds - These interactions occur between positively and negatively charged particles deep within the hemoglobin away from water.
What causes hemoglobin to pick up or release oxygen molecules?
The oxygen binding properties of hemoglobin exist because of the interaction between oxygen and the iron atom of the heme groups and hemoglobin's quaternary structure. However, the ability of hemoglobin to pick up or release oxygen also depends on the pO2, the partial pressure of the oxygen in its environment. When the partial pressure of the oxygen(pO2) is high, as it is in the capillaries around the lung, each molecule of hemoglobin can carry its maximum load of four oxygen molecules. As the blood circulates around the body, the blood experiences lower levels of partial pressure. At these low levels of pO2, the hemoglobin releases some of the oxygen it is carrying. See graph below.
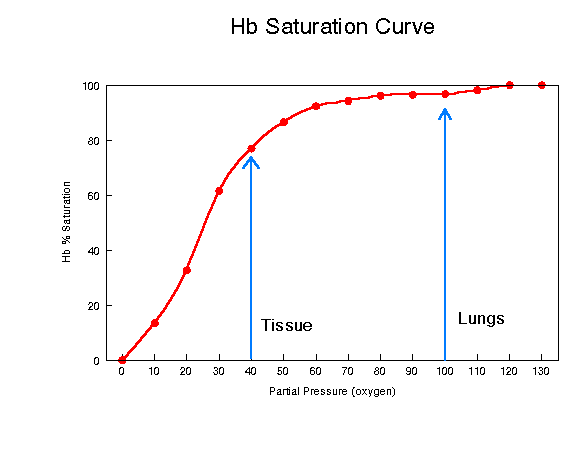
Figure 2: Partial Pressure vs. Hemoglobin/Oxygen Concentration
This graph demonstrates the significant role that partial pressure of oxygen plays in hemoglobin's ability to bind and release oxygen. The graph above shows that the hemoglobin near the lungs is very close to its maximum value for carrying oxygen at a partial pressure of 100mm. When the partial pressure decreases to 40mm, the hemoglobin's oxygen concentration also decreases from close to 100% down to around 75%. This graph was accessed at http://www2.austin.cc.tx.us/~emeyerth/hemoglob.htm.
Bohr Effect
The Bohr Effect is another factor that affects hemoglobin's binding ability. According to http://www2.austin.cc.tx.us/~emeyerth/bohr.htm, normal physiological pH is 7.4. The Bohr effect suggests that if the pH drops below 7.4, the hemoglobin's ability to bind to oxygen will be lessoned. See graph below.
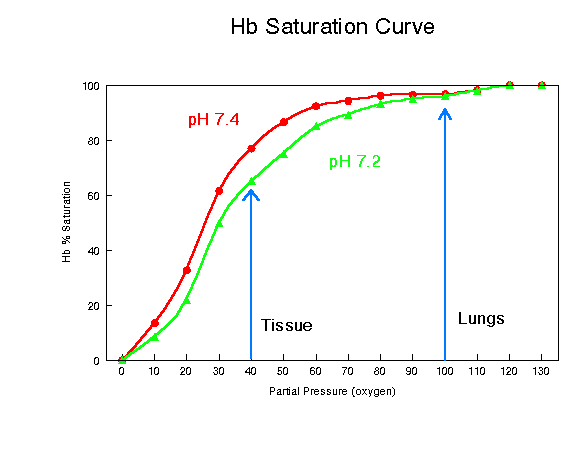
Figure 3: Bohr Effect
The above graph clearly shows that an increase in hydrogen ion concentration(a drop in pH) decreases the amount of oxygen that will bind to hemoglobin at any partial pressure. This graph was borrowed from http://www2.austin.cc.tx.us/~emeyerth/bohr.htm.
Comparing Human Hemoglobin Sequence to that of Other Species

Potential Threats to Hemoglobin
1) Carbon Monoxide - CO binds to hemoglobin with a higher affinity than oxygen. As a result, CO destroys hemoglobin's ability to transport and release oxygen throughout the body. If exposed to too much CO for too long, a person will likely die due to the lack of oxygen be transported to the brain.
2) Sickle Cell Anemia - A sickled red blood cell is caused by a mutation that substitutes a problematic amino acid in either the alpha or beta polypeptide of a hemoglobin. This mutation results in an irregular shaped red blood cell due to hemoglobin forming long rods when giving away oxygen. According to http://www.scinfo.org/sicklept.htm, sickle cell can cause many complications:
1) pain episodes
2) strokes
3) increased infections
4) leg ulcers
5) bone damage
6) yellow eyes or jaundice
7) early gallstones
8) lung blockage
9) kidney damage and loss of body water in urine
10) painful erections in men (priapism)
11) blood blockage in the spleen or liver (sequestration)
12) eye damage
13) low red blood cell counts (anemia)
14) delayed growth
Treatment of Sickle Cell Anemia
According to OMIM,
"stimulating fetal hemoglobin by increasing gamma-globin synthesis in
patients with sickle cell disease would be expected to reduce the formation
of intracellular S polymer and improve the acute and chronic hemolytic and
vasoocclusive complications of the disease.
Conclusion
Hemoglobin is the protein inside red blood cells that makes transporting oxygen throughout the body possible. There are many factors that influence oxygen binding to hemoglobin such as the iron present in the heme groups, the partial pressure of oxygen, ph(Bohr's Effect), and the constantly folding quaternary hemoglobin structure. Click here for a link to a chime model of hemoglobin.
References
Bohr Effect. Austin Community College. Available from: http://www2.austin.cc.tx.us/~emeyerth/bohr.htm.
Hemoglobin. University of Massachusetts. Available from: http://www.umass.edu/microbio/chime/hemoglob/2frmcont.htm
Hemoglobin/Oxygen Binding. Austin Community College. Available from: http://www2.austin.cc.tx.us/~emeyerth/hemoglob.htm
Ho, Chien. Structure of Proteins and DNA. Carnegie Mellon University. Available from: http://www.psc.edu/science/Ho/Ho.html.
Purves, William K. 2001. Life: The Science of Biology. 6th ed. Sinauer Associates, Inc.
Sickle Cell Anemia. 1997. Sickle Cell Anemia Information Center. Available from: http://www.scinfo.org/sicklept.htm.
Davidson College Molecular Biology Homepage
Send questions or comments to brstonestreet@davidson.edu