This web page was produced as an assignment for an undergraduate course at Davidson College.
My Favorite Protein: bFGF / FGF-2
Basic fibroblast growth factor (bFGF, FGF-2) is a member of the FGF family. bFGF has been show to have mitogenic, chemotactic, and angiogenic activity, promoting cell growth, differentiation, and motility (McFarlane et al., 1995; Unsickler et al., 1991; Szebenyi et al., 2001). As FGFs have high affinity for heparin, they are often called heparin-binding growth factors. FGFs are closesly related to the interleukin (IL) protein family, the molecules used as signals by the immune system in between leukocytes (Zhu et al., 1991). In particular, bFGF bears a high degree of similarity to the folds of IL-1.
Structure and Function

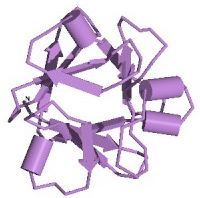
Figure 1. The three-dimensional structure of bFGF, showing the 12 beta sheets. Image from the PDB
bFGF is a 288-residue protein with 12 antiparallel beta sheets (Zhu et al., 1991; Seddon et al., 1991). There are three putative binding regions. Two of these regions, residues 33-77 and 102-129, bind with a moderate degree of affinity to heparin, which protects bFGF from degradation and denaturation while enhacing the binding affinity of the third region with its FGF-receptor (Seddon et al., 1991). Asn-36 and Lys-128 are thought to bind to two of the sulfates in heparin. Although bFGF possess four Cys residues, none of them link together to form a disulfide bond, with two of them existing in a reduced form. The phosphorylation of Thr-121 and Thr-113 by Protein Kinase A greatly enhaces binding to the FGF-receptor via an induced conformational change (Zhu et al., 1991). The exact residues that interact with the FGF-receptor are not known, 115-124 have shown some binding, though are not sufficient to produce a full activation of the cell.
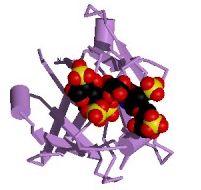
Figure 2. Heparin bound to bFGF. Image from the PDB.
bFGF must bind to an extracellular receptor to enact its effects on the cell; it does not cross the cell membrane. It has been been shown to have profound effects on the development of the nervous system (McFarlane et al., 1995; Unsickler et al., 1991; Szebenyi et al., 2001), and was implicated in regrowth of arterial cells following injury (Reidy et al., 1991). Based on this, bFGF has been under investigation as a form of treatment for myocardial ischemia (PDF file), gliomas, and is used as a marker in several other cancers.
References
McFarlane, Sarah, McNeil, Lisa, Holt, Christine (1995) FGF signaling and target recognition in the developing Xenopus visual system. Neuron 15:1017-1028.
Szebenyi, G., Dent, E.W., Callaway, J.L., Seys, C., Lueth, H., Kalil, K (2001) The Journal of Neuroscience 21(11):3932-3941.
Unsickler, K., Groethe, C. Otto, D., Westerman, R (1991) Basic fibroblast growth factor in neurons and its putative functions. Annals of the New York Academy of Science 68:300-305.
Zhu, X., Komiya, H., Chirino, A., Faham, S., Fox, G.M., Arakawa, T. Hsu, B.T., Rees, D.C (1991) Three-dimensional structure of acidic and basic fibroblast growth factors. Science 251:90-94.
Seddon, A., Decker, M., Muller, T., Armellino, D., Kovesdi, I., Gluzman, Y., Bohlen, P (1991) Structure/Activity relationships in basic FGF. Annals of the New York Academy of Science 68:98-108.
Reidy, M., Lindner, V (1991) Basic FGF and Growth of Arterial Cells. Annals of the New York Academy of Science 68:290-299.