*This website was produced for an assignment in an undergraduate course at Davidson College*
Summary
Since President Obama lifted the ban off stem cell research last March, stem cell research has become increasingly prevalent in the study of human diseases and treatments. The study presented by this paper investigated the genes necessary to induce pluripotent stem cells in both mouse embryonic and adult fibroblasts. To reprogram differentiated somatic cells, their nuclear contents need to be transferred to oocytes or be fused to embryonic stem (ES) cells. Thus, there must be something inherent about unfertilized eggs and ES cells that causes the rebooting of their ability to differentiate into any of the three germ layers. In order to discover these factors, they selected 24 candidate genes ranging from transcription factors specific to embryonic self-renewal to up-regulated genes found in tumors. To select for pluripotent transgenic cells, they used homologous recombination to insert a fusion protein made up of b-galactosidase and neomycin resistance genes into the mouse Fbx15 gene. This gene is specifically expressed in mouse ES cells but is not necessary for the induction of pluripotency or viability. Therefore, only induced pluripotent stem (iPS) cells would be resistant to the addition of G418, since the neomycin resistance gene is necessary for survival and only functioned in embryonic cells. Using retroviral transduction, they introduced varying amounts of the 24 genes of interest into mouse embryonic fibroblasts from embryonic Fbx15 expressing the fusion protein. Mouse embryonic fibroblasts (MEFs) that have been mitotically inactivated are commonly used as a substrate for cells to grow on and secrete many factors necessary for pluripotency.
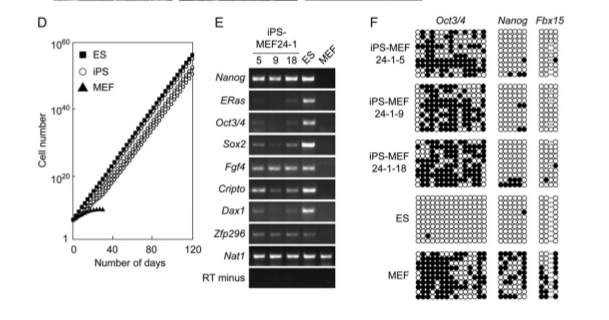
Figure 1
In part A, the assay system they used is depicted with the fusion protein (bgeo) within the Fbx15 locus. The promoter is inactivated until the locus is infected by the cDNA of the pluripotency-inducing candidate genes. The “mock” petri dish in part B shows the lack of resistant colonies with only one factor in contrast to the 22 resistance colonies with all 24 factors found in the right petri dish. Part C demonstrates the difference in morphology among normal embryonic stem cells, iPS cells with 24 factors, and normal mouse embryonic fibroblast cells. Only 5 clones of the 12 clones that they continued cultivating showed similar morphology to ES cells, which includes round shape, scarce cytoplasm, and large nucleoli. A new batch of iPS cells with 24 factors (iPS-MEF24) was produced with another 29 resistant colonies surviving. Only four of the six clones they cultivated exhibited ES cell morphology. They show the growth curve of these selected clones in comparison to normal ES cells and normal MEF cells in part D. The proliferation trend of iPS cells was very similar to ES cells and remarkably different from that of the MEF normal cells. Analysis of ES cell marker genes in the iPS-MEF24 cells using reverse-transcription PCR (RT-PCR)is shown in part E. Nat1, which codes for a detoxifying enzyme, is the positive loading control since it is expressed in all cells. RT minus represents the negative control for comparison. Nanog, Fgf4, Cripto, and Zfp296 exhibit consistent and relatively robust expression of RNA, while ERAs, Oct3/4, Sox2, and Dax1 have weak signals relative to the normal ES cells. The complete lack of any signal of an ES marker gene in the MEF lane except for at the positive control provides sufficient evidence that these marker genes are somewhat expressed in the iPS-MEF clones. Part F is an assay of bisulfite genomic sequencing of the promoter genes of Oct3/4, Nanog, and Fbx15. Only Nanog and Fbx15 are significantly demethylated at the Cpg dinucleotides, indicating the activation of their promoter region. ES cells are the positive control, with all three genes demethylated (since they are all expressed in the embryo). MEF is the negative control, with all three of the genes mostly methylated. Because Oct3/4 remained methylated in the iPS-MEF clones, they postulated that a specific, smaller combination of the 24 factors triggered the expression of ES cell marker genes.

Figure 2
In the bar graph in part A, the y-axis represents the colony number, with white indicating the number after 10 days and black indicating the number after 16 days. The x-axis shows each removal of one factor from the 24 factors in comparison to number of colonies with all factors and a negative control without any factors. Factors 3,4,5,11,14,15,18,20,21, and 22 showed evidence of drastic reduction in colony viability. They then compared the colony number when one factor was removed from the 10 total factors (determined from the previous graph) along with 10 factors, all factors, and no factors. Surprisingly, the presence of the 10 selected candidate genes inferred a greater resistance and colony survival rate than all 24 candidate genes combined. They found that the removal of Oct3/4 (factor 14), Kl4 (factor 20), Sox2 (factor 15) resulted in little or very few colonies in figure B. While colonies did survive without c-myc, their morphology was flat and very different from that of normal ES cells. Thus, they concluded that these four genes were necessary and sufficient to induce pluripotency in differentiated cells. In part C, significant colony growth was not seen with the removal of any one of these factors and no growth was seen with only 2 factors present. Part D compares the morphology of iPS cells from a clone with 4 factors, those from a clone with 10 factors, and those from a clone with Sox2 removed from the selected 4 factors. The cells with only three factors displayed rough surfaces, while the other two iPS clones were relatively similar in shape.
Figure 3
The RT-PCR gel in part A is similar to that in 1E but instead iPS-MEF3,4 and 10 clones ES marker genes are analyzed. Researchers used primers that would solely amplify endogenous genes. Again, Nat1 and RT minus are the positive and negative controls, respectively. The amount of ES marker genes in all three types of clones varied extensively among each type; however, the majority of ES marker genes were expressed in iPS-MEF4 and iPS-MEF10, except Ecat1. Interestingly, iPS-MEF3 showed higher expression of the ES marker genes ERas, Fgf4, and Cripto. There must be something about Sox2 (the factor missing) that inhibits the expression of those three characteristic ES genes in the other clones that include this factor. In order to look at the promoter activity of Oct3/4 and Nanog, they performed a chromatin immunoprecipitation analysis using real-time PCR in part B. They found that histone acetylation increased and dimethylation decreased in both gene promoters in iPS-MEF4 and iPS-MEF 10 clones as compared to normal MEF cells. The activity of these promoters indicated increased access to transcription factors and increased expression of these ES genes. Part C is another bisulfite sequencing analysis of iPS-MEF4 and iPS-MEF10. Fbx15 was completely demethylated, while Oct3/4 and Nanog remained partially methylated. This demonstrates the striking similarity between iPS cells and ES cells but also that the two are not identical. SSEA-1 (stage-specific embryonic antigen-1) and alkaline phosphatase are both indicators of undifferentiated cells. In part D, antibodies specific for the two bind to both iPS-MEF4-7 and iPS-MEF10-6, but more so to the iPS-MEF10-6 clone.
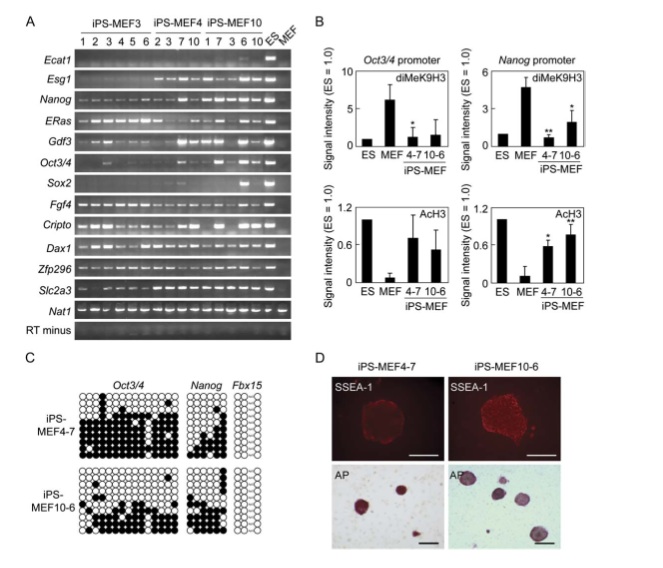
Figure 4
In part A and B, a DNA microarray of iPS cells, MEF cells, ES cells, and several variations of MEF cells revealed their global-gene expression patterns. Part A was evaluated by a Pearson correlation analysis, which measures the dependence between two quantities (the cells involved). It appears that iPS cells are clustered near to ES cells and not with fibroblasts and the variations of fibroblasts. The genes that are upregulated (red) are depicted in part B. The first area designates genes upregulated in ES cells and iPS cells, the second area designates genes upregulated more in ES cells and iPS cells with more than 3 factors, and the third area designates genes upregulated more in solely ES cells. The lack of genes in the second area upregulated by iPS cells with three factors may locate the reason behind their lack of pluripotency. Again, iPS cells are very similar to ES cells (much more than they are to their fibroblast derivatives) but at the same time, not identical.
Figure 5
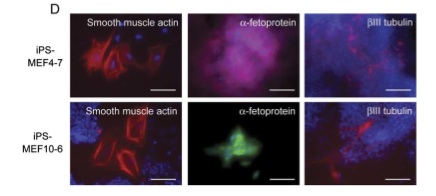
Teratoma formation can be used to look at the ability of a cell to differentiate into three germ layers. They successfully obtained tumors from subcutaneously injecting 5 iPS-MEF10 clones, 3 iPS-MEF4 clones, 1 iPS-MEF4wt clones (c-myc wild-type), and 6 iPS-MEF3 clones. Figure A and B depict histological evidence of pluripotency of iPS-MEF4-7 clone. This clone successfully differentiated into cartilaginous, neural, and epithelial tissue, which represent all three germ layers. Although 3 factors in iPS cells were able to trigger expression of several ES marker genes, they were not sufficient to induce pluripotency and remained differentiated in part C, bottom panel. IPS-MEF 4 and iPS-MEF10 clones were able to differentiate from embryoid bodies in the top panel to various tissues seen in the bottom panel of part C. In part D, fluorescent dyes give a closer look at the differentiated tissue of IPS-MEF 4 and iPS-MEF10. A mesoderm marker (a-smooth muscle actin), endoderm marker (a-fetoprotein), and an ectoderm marker (bIII tubulin) give compelling evidence for the pluripotency of both clones and the nullipotency of the iPS clones with only 3 factors.
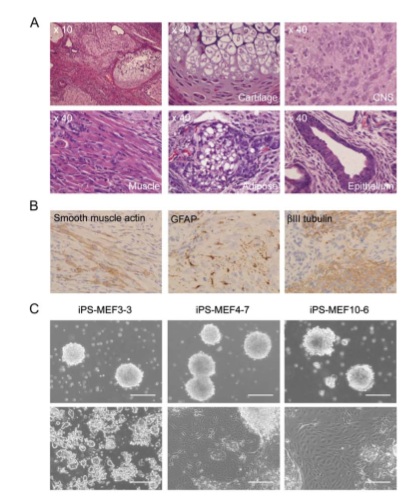
Figure 6
In order to look at the ability to induce pluripotency in adult cells, researchers used tail-tip fibroblasts (TTFs) with the same fusion protein construct. They were able to obtain 3 drug-resistant colonies with four factors from four 7-week old male mice (iPS-TTF4). In addition, they obtained 13 drug-resistant colonies from a 12-week-old female with the Fbx15 fusion protein construct, which also has a CAG promoter and expresses green fluorescent protein (iPS-TTFgfp4). Part A demonstrates the resemblance between these adult iPS cells and ES cells. An RT-PCR analysis of iPS-TTFgfp4 revealed that the majority of ES marker genes were expressed but the level of expression varied among clones. Once again, Ecat1 is missing from all iPS clones. In figure C, 2 iPS-TTFgfp4 clones were microinjected into blastocysts and obtained 18 embryos, 2 of which expressed green fluorescent protein (bottom panel). Part D provides evidence that four factors are sufficient and necessary to induce adult mouse fibroblast differentiation into three germ layers through histological staining with GFP antibody.
Figure 7
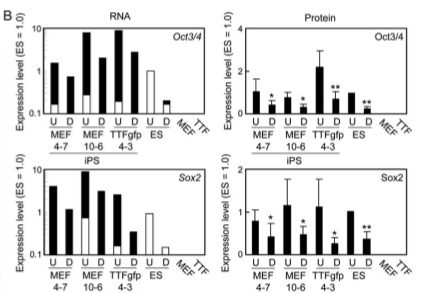
The Western blot in part A demonstrates the similarity in protein production between iPS-MEF cells, iPS-TTFgfp4, and ES cells. b actin protein is the positive control. Both Nanog and ERas proteins were present in both iPS-MEF and iPS-TTFgfp and especially prevalent in the iPS-MEF10 clone. In contrast, the ES cell level of Nanog and ERas proteins was much greater than those of iPS cells. The level of p53 protein (a tumor suppressor) was lower in iPS cells and ES cells than in MEF cells, as expected. In part B, RNA and protein levels in iPS-MEF cells, iPS-TTFgfp cells, ES cells, MEF cells, and TTF cells were compared between undifferentiated (U) and induced differentiated (D) and between Oct3/4, Sox2, and Nanog. While RNA levels of Oct3/4 and Sox2 in undifferentiated and differentiated iPS cells were higher than that in ES cells, the protein level showed similar drops in expression between iPS cells and ES cells. In order to look at the role that retroviral insertion played in induction of pluripotency, they performed a southern blot of each clone using a Klf4 cDNA probe in part C. It is clear that each clone exhibits a distinct transgene pattern of integration. They then looked at the karyotype of the iPS-TTFgfp4-2 clone for chromosomal alterations in part D and observed no abnormalities. Finally, in order to rule out the possibility that iPS cells were not just a “contamination” of pre-existing ES cells, they removed the feeder cells used previously to see if differentiation occurred, with or without LIF (Leukemia inhibitory factor, absence induces differentiation but maintains pluripotency). The ES cell is the only cell that remains undifferentiated in the presence of LIF, while iPS-MEF4-7 and iPS-TTFgfp4-3 differentiate without the presence of feeder cells. ES cells, iPS-MEF4-7, and iPS-TTFgfp4-3 differentiate without LIF and feeder cells. This is sufficient evidence that iPS cells are not merely “contamination” of pre-existing ES cells.
Critique
The authors of this paper present a great deal of data in a manageable and informative way. They use a variety of experimental methods to present their findings and thoroughly convince their readers that their claims are accurate. The figures are detailed and extensive. Gel analyses were easily comparable and credible because of the consistent presence of a clear negative control (RT minus) and a constant protein loading level in their positive control (Nat1). In addition, a lane was provided for ES cells and MEF cells which allowed straightforward assessment of the data. Everything was clearly labeled and comprehensible. The histological evidence for comparison of ES cell and the various iPS cell combinations was compelling and substantiated their assertion that iPS cells were similar but not identical to ES cells. The fluorescence microscope analysis of embryos expressing GFP was especially striking.
Additionally, the authors make a great effort not to overstate their data or their claims. What was somewhat troubling in the beginning of this experiment was the small amount of iPS cells they produced. In addition, the expression of ES marker genes was not consistent among clones of the same type. In the discussion, however, the authors comprehensively address this issue and give plausible reasons for such findings. They point out that there may be a narrow threshold above and below a certain level of the factors that can induce viable, pluripotent cells. This statement is substantiated by the research showing that a 50% increase or decrease in Oct3/4 protein can trigger differentiation. They point out that the low level of necessary proteins compared to the RNA level reveals an underlying mechanism of protein regulation and would also explain the lack of iPS clones. Another possibility they introduced for this low result could be that chromosomal modifications are necessary for the production of a substantial amount of iPS clones, since the karyotype they performed was not sufficient to rule out chromosomal alterations. Finally, they call attention to the suggestion that there may be more factors that they have missed and leave room for future findings.
One issue with this paper was the histological analysis in figure 6A. They claim that the morphology of iPS-TTFgfp4 cells is “indistinguishable” from ES cells. However, the morphology of ES cells in figure 1C is not identical to figure 6A. Also, figure 5D seems like it could be a remarkable illustration of the differentiation ability of the iPS cells but it was hard to understand what the reader is looking at and what he or she is supposed to notice, especially when they use two different colors to stain the endoderm marker. Other than these two figures and the discrepancies that they themselves point out in their discussion, this study has a sound argument and strong data to back it up.
Future Experiments
One of the issues presented in this paper was the random integration of the transgene that may have caused silencing or a fusion with endogenous genes. In order to prevent these potentially lethal insertions, another method of transduction is necessary. Future studies should look at whether the transformation of somatic cells by these four genes necessitates their injection into the cell’s genome. Perhaps a type of virus that does not need to incorporate itself into its host could be introduced and the induction of pluripotency could be observed. This is plausible because the supplemental data showed that total expression level of the four genes was greater in iPS cells than ES cells and total protein amount in iPS cells was comparable to ES cells, indicating the silencing of transgenes by some mechanism. Thus, the transgenes may not be necessary after initial introduction in maintaining the pluripotency of the cell.
In order to test this hypothesis that the transgenes are unnecessary after a period of time, an inducible promoter that is controlled temporally could provide evidence for the maintenance of pluripotency by the endogenous genes or transgenes. In the presence of doxycycline, a Cre-loxP system is induced by the tetracycline reporter gene. If the transgenes were necessary, the iPS cells would differentiate because they were excised by the Cre-loxP system.
Understanding the mechanisms behind the action of and interaction between Oct3/4, Klf4, Sox2, and c-myc could prove helpful in streamlining the reprogramming process. Therefore, a yeast-two hybrid system could be utilized to reveal some crucial aspects of this coordination. Using GFP as a reporter gene, the GAL4 DNA-binding domain could be fused to one of these required proteins and the GAL4 activating domain fused to another one of these proteins or relevant DNA fragments. Interaction between the two fused objects would result in expression of GFP. In addition, fluorescence recovery after photobleaching (FRAP) could be performed in order to understand where these proteins are in the cell over a period of time.
Future potential experiments should most importantly look at the ability of Oct3/4, Klf4, Sox2, and c-myc and other factors in reprogramming different cell types within the mouse and eventually human cells. Subsequently, the ability to safely reprogram human cells into undifferentiated cells has exciting applications for a wide variety of human diseases.
References/Links
Takahashi, K. and Yamanaka, S. 2006. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 126: 663-676.
Davidson College Molecular Biology Daily Schedule
For questions or comments, please contact Brianna Pearson