* Permission pending from Saccharomyces Genome Database *
Background Information
ADH1 is the gene that is responsible for producing alcohol dehydrogenase in Saccharomyces cerevisiae, baker’s yeast. Alcohol dehydrogenase is an important component of Saccharomyces cerevisiae's fermentation process. Fermentation is the required for the production of energy, in the form of ATP, in organisms that are inhabit an environment that is oxygen deprived. This anaerobic process involves one molecule of glucose, a sugar,where it is converted into two molecules of carbon dioxide and two molecules of ethanol. Simultaneously, two molecules of ADP are converted into two molecules of ATP. Alcohol dehydrogenase plays an intermediate role by helping to convert glucose into two molecules of pyruvate. Additionally, alcohol dehydrogenase converts two acetaldehyde molecules into two molecules of ethanol.
Homology and Conservation of Gene Across Species
The genetic conservation is very high for ADH1. The e-values for each of the holmologs, both mammalian and invertebrate, were very high when the Blastn was preformed. The results of the Blast searches can be viewed by clicking below.
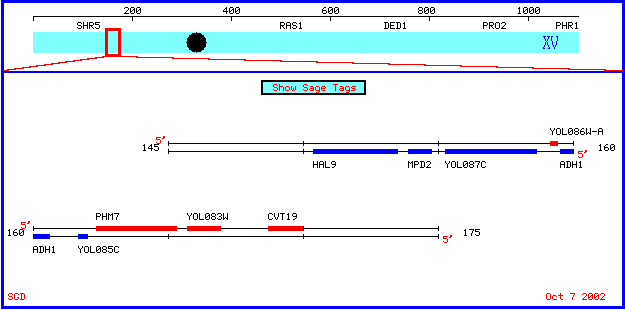
Location of ADH1 in Genome of Saccharomyces cerevisiae
The ADH1 gene is located on the fifteenth chromosome of the Saccharomyces cerevisiae genome. View map below from the Saccharomyces Genome Database :
* Permission pending from Saccharomyces Genome Database *
Protein Information and Structure
The ADH1 gene codes for alcohol dehydrogenase, NAD +, which is a vital component of the fermentation process in Saccharomyces cerevisiae. The SwissProt Database lists alcohol dehydrogenase as being 348 amino acids long and weighing 36692 Da in Saccharomyces cerevisiae. (SwissProt Database) A search of Predator yielded a analysis of the structural make up of alcohol dehydrogenase : 24.14% of the protein is alpha-helices; 24.84% of the structures are extended strands; 54.02% of the structure is random coiling. (Predator Search) A Kyte-Doolittle hydropathy plot analysis showed that there is a small hydrophobic region from amino acid number 150 to 165, showing that alcohol dehydrogenase is a integral membrane protein.
Phenotype Information
Phenotypically a systematic deletion in the ORF ADH1 results in a viable organism. (Abstract : Giaever G, et al, 2002). Alcohol dehydrogenase is an important protein to have in order to perform normal respiration in anaerobic organisms. Therefore it makes little sense that a deletion of in the ADH1 reading frame would allow for a viable organism. Thus i must be true that ADH1 is not solely responsible for the production of alcohol dehydrogenase, or that there are other genes that produce alcohol dehydrogenase.
Conclusions
Alcohol dehydrogenase is a integral component of the fermentation process, and especially important to understand when studying anaerobic organisms. Though deletions in the ORF of ADH1 results in a viable organism, this does not imply that ADH1 is only a novelty. Polygenic traits are very complex and not easily understood and needs to be scrutinized very thoroughly.