This web page was produced as an assignemt for an undergraduate course at Davidson College

Permission pending
The premise of this investigation is that amyloid β (Aβ) species accumulate in the brain and are one of the causes of Alzheimer’s Disease (AD). Additionally, other studies found that proton pump inhibitors (PPIs), such as Lansoprazole, can alter the cleavage of amyloid precursor proteins (APPs) to produce increased levels of Aβ species. The purpose of the investigation was to examine the effects of Lansoprazole on production of Aβ species in both AD cell models and AD animal models.
To begin, the researchers treated AD cell models (PS70 cells) with different concentrations of Lansoprazole. The researchers also tested the effects of other PPIs on the AD cell models. All of the PPIs showed an increase in Aβ40 and Aβ42 production. The researchers then treated cells in both Lansoprazole and R-flurbiprofin (known to reduce Aβ42 production) simultaneously. These cells produced abnormal amounts of Aβ42, which indicated that Lansoprazole is an inverse Υ-secretase modulator.
The researchers then tested the effects of Lansoprazole on BACE1, a protein responsible for the cleavage of APP into sAPPβ, which Υ-secretase turns into Aβ peptides. BACE1 competes with α-secretase for the first cleavage of APP. Only when BACE1 cleaves first are Aβ peptides produced. The researchers found that BACE1 activity was increased in the presence of Lansoprazole, resulting in greater production of Aβ peptides.
Finally researchers tested wildtype and triple AD mutant mice with different concentrations of Lansoprazole. They found that Lansoprazole caused statistically significant increases in Aβ40 production after 5 consecutive days of exposure to 100kg/mg in wildtype mice and statistically significant increases in Aβ40 after 5 consecutive days of exposure to 100kg/mg and 20kg/mg in triple AD mutant mice.
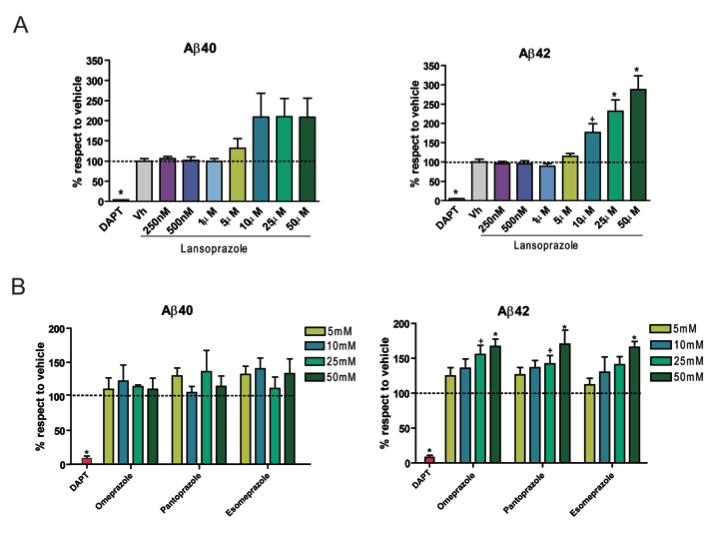
Panel A – This graph shows the effect of Lansoprazole on the amount of amyloid β 40 (Aβ40) in an Alzheimer’s Disease like cells. The bar on the left serves as a negative control. The second bar on the left is the control for the method of administering the drug Lansoprazole. The following bars show amount of Aβ40 produced for each given concentration of Lansoprazole. The presence of 10-50 μM of Lansoprazole caused a two-fold increase in Aβ40 production. This graph shows that exposing an AD like cell to Lansoprazole in concentrations exceeding 10 μM can double the production of Aβ40, which is hypothesized to be one of the causes of AD.
Panel B – This graph shows the effects of Lansoprazole on the amount of Aβ42 production in AD like cells. The cells exposed to DAPT were negative control cells and the cells exposed to the vehicle control for the increase in Aβ42 production caused by the drug delivery method. The production of Aβ42 is dependent on the amount of Lansoprazole the cells were exposed to, the higher the concentration of Lansoprazole the greater the production of Aβ42.
Panel C – This graph is showing the same experiment as panel a: the amount of Aβ40 produced for a given level of PPI except in this graph three different PPI’s are used; Omeprazole, Pantoprazole, and Esomeprazole. All three of these PPI’s failed to produce statistically grater amounts of Aβ40 than the vehicle control.
Panel D – This graph shows the effects of Omeprazole, Pantoprazole, and Esomeprazole on the levels of Aβ42 in AD like cells. The controls in this graph are the same as the previous three. Aβ42 levels are dose dependent, 50μM of each PPI showed the greatest level of Aβ42, but 25 μM also showed statistically grater levels of Aβ42 for Omeprazole and Pantoprazole.
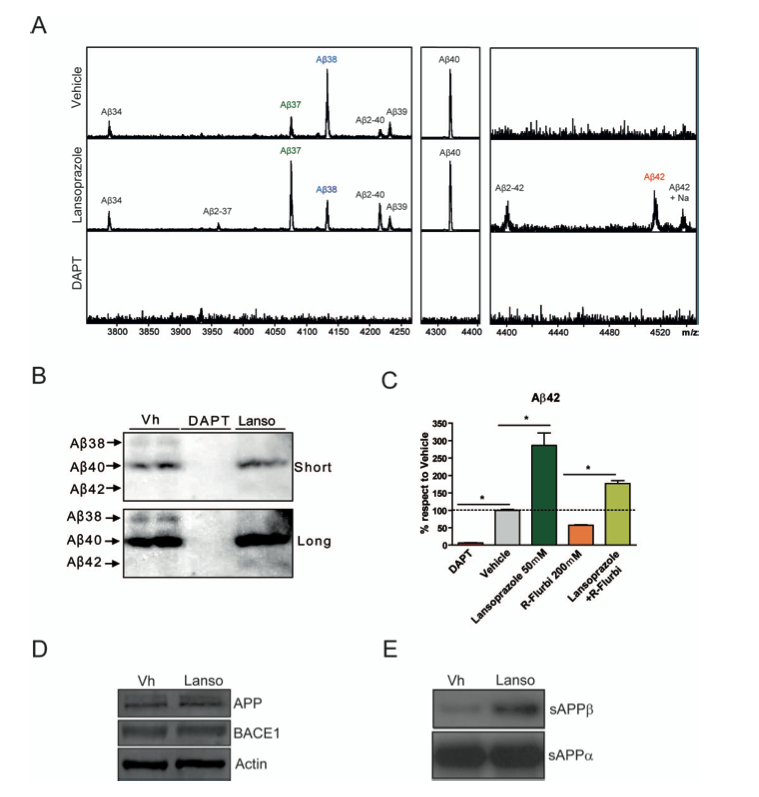
Panel A – The purpose of this graph is to show that Lansoprazole increases the amount of Aβ42 in PS70 cells. Aβ42 was experimentally measured using immunopercipitation. This shows that Lansoprazole does have an affect on the levels of Aβ42 and it is not just the vehicle that is causing the increase in Aβ42.
Panel B – This image is a western blot of Aβ38, Aβ40, and Aβ42 from cells exposed to the vehicle, DAPT, or Lansoprazole. Both short and long time periods are shown. We can see that the amount of Aβ38 is lower in the Lansoprazole than in the vehicle and that the amount of Aβ42 is greater in the Lansoprazole than in the vehicle. This is just another method to quantify the differences in Aβ production in cells exposed to Lansoprazole.
Panel C - This graph shows the inverse γ-secretase modulator (iGSM) activity in the presence of a straight γ-secretase modulator (GSM): R-flurbiprofen. The first two bars are controls: DAPT is a negative control to sow that APP can be repressed and the vehicle controls for the effect the method of drug administration has on the cell.
The third and fourth bars show the effects of Lansoprazole and R-flurbiprofen on the cells respectively. Lansoprazole shows an increase in Aβ42, which is indicative of an iGSM and R-flurbiprofen shows a decrease in Aβ42, which is indicative of a GSM. When cells are exposed to both Lansoprazole and R-flurbiprofen at the same time, they show a moderate increase in Aβ42 levels, which indicates that the iGSM effects are strong enough to counteract the GSM effects of the GSM R-flurbiprofen.
Panel D – This image is a western blot that shows the relative levels of APP, BACE1, and actin in cells that would receive a Lansoprazole treatment and cells that would not receive the treatment. The concentrations of each protein for both treatments are the same, which demonstrates that any change in sAPPβ or sAPPα levels in the cell were due to the Lansoprazole treatment and not random differences in the levels of α-secretases and γ-secretases in the cells. This image is a control for panel E.
Panel E – This image is a western blot that shows relative levels of sAPPβ and sAPPα. The western blot shows that Lansoprazole increases the amount of sAPPβ while levels of sAPPα remained the same in the presence of Lansoprazole. This indicates that Lansoprazole enhances the activity of BACE1 because sAPPβ is the product of APP cleavage by BACE1.
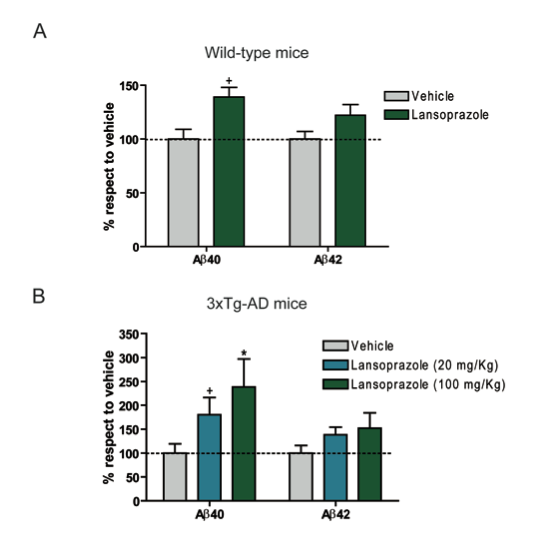
Panel A – This graph shows the effects of 100kg/mg doses of Lansoprazole for 5 consecutive days on Aβ40 and Aβ42 levels of wildtype mice. The graph shows that Lansoprazole increases the levels of both Aβ40 and Aβ42 over control levels, however the increase of Aβ42 was not statistically significant.
Panel B – This graphs shows the effects of 20kg/mg and 100kg/mg doses of Lansoprazole for 5 consecutive days on triple transgenic AD mice (3xTg-AD). While both doses show increases of Aβ40 and Aβ42 for both dosages, only increases Aβ40 levels were statistically significant. Neither dosage of Lanzoprazole had a statically significant effect on Aβ42 levels.
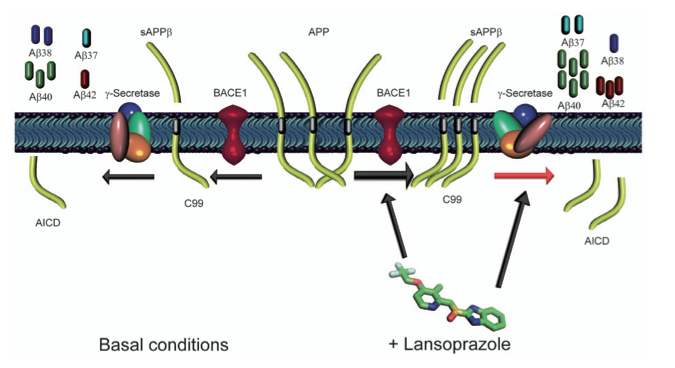
This image depicts the cleavage of APP both in the presence and absence of Lansoprazole. Beginning in the middle, with APP, and proceeding to the right, the image shows that Lansoprazole binds to BACE1 and Υ-secretase. When Lansoprazole binds to BACE1 it increases the activity of the protein, shown by the greater number of APP molecules being processed by the protein. The sAPPβ molecules are then cleaved again by Υ-secretase, which has undergone a shape changes due to the presence of Lansoprazole, causing the protein to create an abnormal amount of Aβ37, Aβ40, and Aβ42 molecules and fewer Aβ38 molecules.
Following the image from the center to the left depicts the activity of BACE1 and Υ-secretase in the absence of Lansoprazole. BACE1 has a lower level of activity shown by the single APP molecule being cleaved. Υ-secretase is depicted in a normal conformation, which results and the standard amount of Aβ37, Aβ38, Aβ40, and Aβ42 molecules.
The researchers concluded that PPIs can increase the production of Aβ species in vitro, which has serious implications for medicine. While PPIs have their medicinal uses, it seems that they also promote the accumulation of Aβ species, which is inadvertently promoting the onset of AD. Additionally, the inappropriate prescription of PPIs by doctors to dementia patients can exasperate their condition by fostering the development of AD.
I found this paper to be very straight-forward and understandable, which is a pleasant change from the challenging nature of most scientific papers. All of their experiments have appropriate controls and their data compelling. The researchers do a good job of not overstepping their data, all of their claims are supported by the data. Although they do state that PPIs cause an increased buildup of Aβ species, they recognize that Aβ species are not a proven cause of AD, they are only associated by correlation. Thus while they claim their study illuminates the dangers of PPIs, they do recognize that they do not fully understand how instrumental PPIs are in the development of AD.