
This web page was produced as an assignment for an undergraduate course at Davidson College.
Review of Optical Control of Muscle Function by Transplantation of Stem Cell-Derived Motor Neurons in Mice by Bryson et al. 2014
Figures are from Bryson et al. 2014
Summary and Review
Bryson et al. concluded that embryonic stem cells can become functional motor neurons in mice. Embryonic stem cells were transfected in vitro to add genes that would cause the cells to differentiate into motor neurons. It took 35 days after transfection for the motor neurons to mature. At maturation, the motor neurons could respond to a stimulus by firing an action potential. Next the motor neurons were transplanted near the cut sciatic nerve of adult mice, which is a peripheral nerve in the hind limb. Motor neurons could respond to pulses of 470-nm blue light, because they contained channelrhodopsin-2, a light sensitive ion channel. This method was called optical control. In addition, the differentiated cells contained glial-derived neurotrophic factor to promote cell survival. Fluorescent transgenes were used to show that the cells were neurons. After the sciatic nerve was severed, some neurons regenerated, which were used as the control. Functions of the motor neurons from stem cells were compared to the functions of endogenous regenerating motor neurons. The researchers found that the grafts of motor neurons into hosts were successful and the motor neurons were functional. Motor neurons were able to grow into different muscles surrounding the sciatic nerve and respond to electrical or optical stimulation. The goal was to cause muscle contractions of different sizes and at different times to imitate endogenous motor neuron activity in various muscle types.
The fluorescence micrographs were beneficial for illustrating the proteins present in the cells to prove the identity and characteristics of the motor neurons. It would be interesting to learn more about the protocol used to differentiate the stem cells into motor neurons, specifically which genes need to be expressed and the necessary transcription factors. The authors cited other papers as the source for this method. The graphs showing muscle stimulation provided quantitative data that the motor neurons were functioning in different muscles in response to electrical or optical stimulation. In some experiments the sample size was small (n = 4), but the experiments were replicated and error bars in graphs illustrated variation, which make the data more credible.
Data presented in this paper suggest that in the future motor neurons and possibly other cell types derived from stem cells may be functional in humans. The experiments presented in this paper show that embryonic stem cells became motor neurons that could innervate muscles and respond to a stimulus. Differentiating stem cells into cells that can form a structure is less challenging than developing functional neurons, which receive and send signals to other parts of the body. This research was “proof of concept” work that neurons derived from embryonic stem cells can function in live animals. The authors provided background information about previous attempts to repair neurons and explained the importance of their research questions. Stem cells are a popular research topic and the results of this paper provide support for stem cells as a source of cells to repair damaged neurons. Repairing neurons has therapeutic potential for individuals suffering from nerve damage resulting from either diseases or injuries. The results presented in this paper are promising for future clinical trials in humans. It was important to show that the stem cells could differentiate into functional neurons, which was demonstrated with multiple experiments. A major challenge in regenerating neurons is successfully connecting them to the central nervous system. The authors explain that before this research can be used in humans, future experiments must be done to determine how an optical stimulator inside a test subject can stimulate the motor neurons so nerves are not exposed. Another challenge is that cells can be damaged by short wavelengths of light. Red variants of channelrhodopsin-2 may be better for cell survival. Perhaps another area to investigate would be if the same functional properties occur with motor neurons derived from induced pluripotent stem cells.


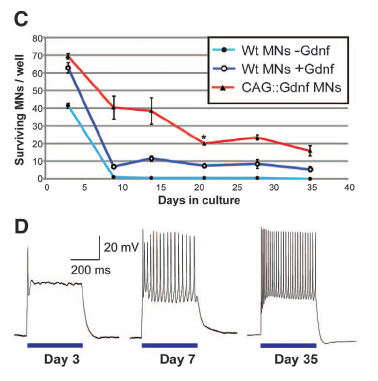
Figure 1: Embryonic stem cells were successfully differentiated into functional motor neurons. The fluorescence micrographs in Part A compare parental cells to the transgenic experimental cells. All embryonic stem cells were stained with Isl1/2 (red), which shows that they have proteins that are expressed in motor neurons. Parent cells contained a motor neuron-specific reporter (green), which was retained in the differentiated motor neurons with added genes. The blue stain indicates that the experimental cells contain channelrhodopsin-2. Part B shows immunostaining of the glial-derived neurotrophic factor (Gdnf) protein. Cells with Gdnf show the protein as red. Motor neurons derived from stem cells that produce Gdnf have a higher survival rate over a period of 35 days than wild type cells with or without Gdnf, which was found by measuring the survival rate of hundreds of cells (C). Differentiated motor neurons with channelrhodopsin-2 and Gdnf respond to an optical stimulus, which is shown as the blue horizontal lines (D). The cells have an action potential that increases in size and frequency until the cells reach maturity at 35 days. The scale in Part D indicates the length of time in milliseconds and the size of the response in millivolts.
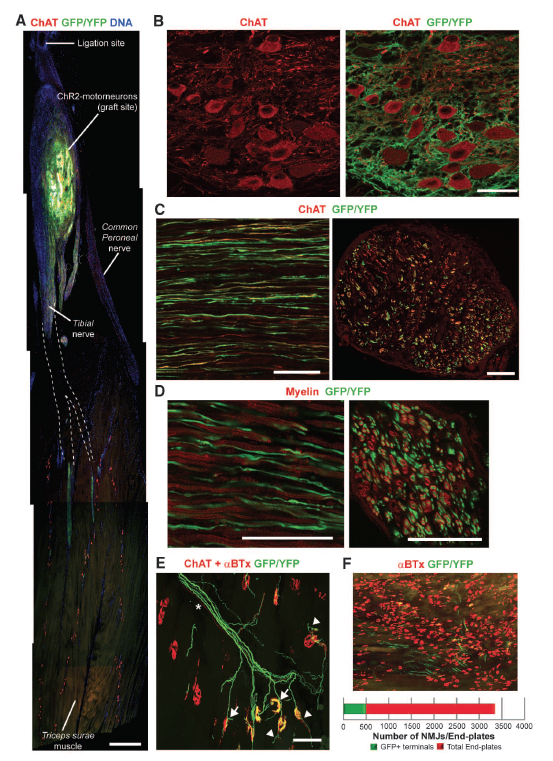
Figure 2: The fluorescence images from a confocal microscope show that motor neurons with channelrhodopsin-2 grow axons and innervate muscles in mice. Immunostaining shows the presence of specific proteins in the cells. The image of a hind limb in Part A shows that the graft survived in the host muscle and axons are predicted to grow (A). Motor neurons from embryonic stem cells look like adult motor neurons based on their expression of choline acetyltransferase (red), which is found in mature motor neurons (B). Endogenous motor neurons and those derived from stem cells are present and growing axons (C). In Parts C and D the left image is a longitudinal section of the neurons and the right image is a transverse section. Staining for myelin indicates that the axons are myelinated and will function like endogenous motor neurons (D). Even though axons were growing, based on the structure of the axons the researchers concluded that some neurons were not functioning (E). Part F shows the parts of a muscle that were innervated by comparing the number of neuromuscular junctions to end plates.
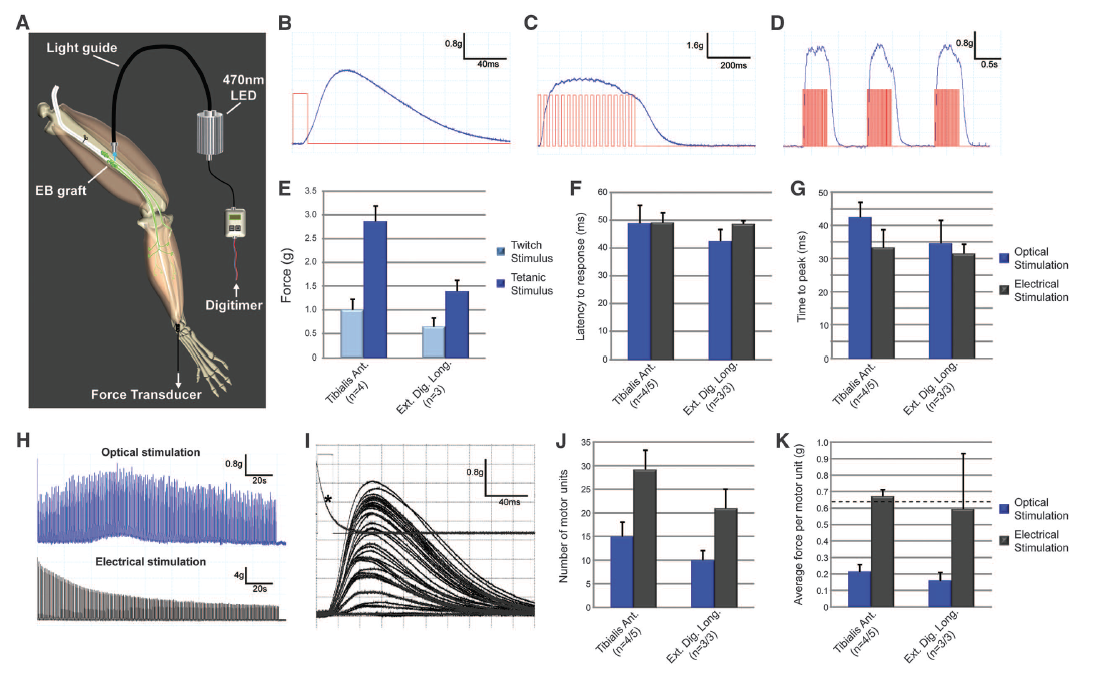
Figure 3: The results presented in this figure show that optical stimulation causes stem cell derived motor neurons to function in mice. Part A depicts a schematic diagram of the methods used to stimulate muscles using a light-emitting diode (LED). Light pulses lasting different amounts of time caused muscle twitches and contractions (B and C). The red lines in Parts B and C were the stimulus and the blue lines on the graphs were the muscle response. Muscle responses were lost after the stimuli stopped, but recurring stimulation could cause repeated responses. This showed that the muscle contractions were not isolated events (D). Different muscle types can have muscle contractions with varying amounts of force (E). Graphs F and G compare optical and electrical stimulation in two different muscle types, measuring time to initial response and time to peak response in milliseconds. The bar graphs show that the different stimuli had the same effects in each cell type, which suggests that endogenous and stem cell derived motor neurons have similar response capabilities. Next the researchers tested if a motor neuron could respond to more than one stimulus before reaching a fatigue state. Motor neurons from stem cells exposed to repetitive optical stimulation are fatigue resistant, while endogenous motor neurons loose the ability to respond to electrical stimulation (H). Activation thresholds for motor units vary (I). The type of stimulation the motor neurons responded to indicated their origin, because the endogenous motor neurons were activated by electrical stimuli and the motor neurons from stem cells responded to optical stimulation. Graph J shows that about half of the motor units in two different muscle samples were motor neurons from stem cells. Endogenous motor neurons (black bars) were able to exert more force in response to a stimulus than the motor neurons from stem cells (blue bars).
Genomics Page
Biology Home Page
© Copyright 2014 Department of Biology, Davidson College, Davidson, NC 28035