This web page was produced as an assignment for an undergraduate course at Davidson College.
Summary:
Lactobacillus
plantarum and
Juvenile Growth
The
research paper entitled "Lactobacillus plantarum strain
maintains growth of infant mice during chronic undernutrition" was
published by Schwarzer et al. in the journal Science on
February 19th, 2016 (Schwarzer et al., 2016). This paper
sought to elucidate the previously unknown role of the intestinal
microbiota in juvenile growth. The researchers found that specific
lactobacilli strains promote juvenile growth and protect against
stunted growth caused by undernutrition. These findings have major
implications on the potential prevention of stunted human growth in
malnourished treatment by microbial intervention. This paper presented
logical and thorough experiments to investigate its hypotheses. Each
conclusion presented in the paper was well supported from the given
data. Additionally, each figure was clearly presented with useful
labels and simple designs. My major complaint is with the flow of the
manuscript, which jumps from figure to figure instead of introducing
the data sequentially. A rearrangement of the figures or the
manuscript could have alleviated this problem and improved readability.
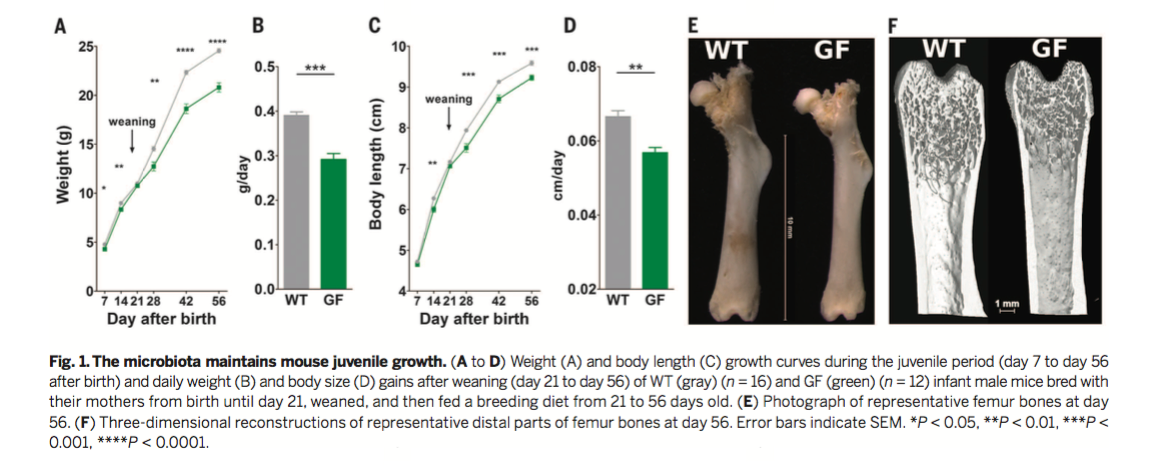
Figure 1
To identify the general role of the microbiota in
juvenile growth, wild-type (WT) and germ-free (GF) mice were fed a
standard diet until 8 weeks of age (56 days after birth). Despite
similar amounts of food consumption (as shown in a supplementary
figure), WT animals exhibited greater body weights and body lengths
than GF animals. Figure 1A displays body weight over time, and Figure
1C displays body length over time for both WT and GF mice. Figure 1B
and 1D are quantifications of part A and C, respectively. The
researchers observed statistically significant differences in both
body weight and body length after weaning between WT and GF mice.
Despite these differences, GF and WT animals demonstrated no
significant differences in adiposity, cortical bone density, or leptin
levels (from the supplementary figures). Figures 1E and 1F display
representative femur bones of WT and GF mice. The researchers found
that several bone growth parameters, including femur length and
cortical thickness, were reduced in GF mice. Together, these data
suggest that the microbiota is an active component in stimulating
juvenile growth.

Figure 2
Mammalian growth is by the somatotropic axis, a hormonal system in which growth hormone (GH) stimulates the liver and other tissues to secrete insulin-like growth factor-1 (IGF-1) which in turn stimulates growth. To determine whether the microbiota maintains the activity of the somatotropic axis, serum levels of GH, IGF-1, and IGFBP-3 (an IGF-1 carrier protein) were determined by ELISA (Enzyme-Linked Immunosorbant Assay). Expression levels of Igf-1 and Igfbp3 were determined in the liver by qRT-PCR.The researchers did not observe significant differences in serum GH between WT and GF mice (Figure 2A). However, GF mice exhibited a significant reduction in IGF-1 and IGFBP-3 at multiple time points (Figures 2B and 2C). Igf-1 and Igfbp3 transcript levels were also significantly reduced in GF mice (Figures 2D and 2E).
Phosphorylation of AKT serves as a marker of IGF-1 receptor (IGF-1R) activity, a downstream marker of the somatotropic axis.The ratio of phosphorylated AKT (pAKT) to total AKT (tAKT) was determined by densiometric analysis of Western Blot data. Representative Western Blots of WT and GF animals are displayed in Figure 2F along with the ratios of pAKT to tAKT for the two groups. The researchers found increased phosphorylation of AKT in WT mice, indicating greater somatotropic axis activity in WT mice. Taken together, these data provide evidence that the microbiota modulates the activity of the somatotropic axis. This finding alludes to a potential mechanism of microbiota-induced juvenile growth, which is further explored in a later figure.

Figure 3
From here, the researchers characterized the contribution of the gut microbiota to juvenile growth under the condition of chronic undernutrition. For the undernutrition treatment, animals were fed a non-standard animal chow with low protein, fat, and vitamins. Figure 3A shows that after weaning at Day 21, mice receiving the nutritionally depleted diet demonstrated a dramatic reduction in body weight. GF mice receiving the nutritionally deficient diet demonstrated a greater reduction in body weight than WT mice. Figure 3C shows similar reductions in growth in response to the depleted diet as measured by body length. Once again, GF mice demonstrated a greater reduction in body length in response to undernutrition. Whole mice and femurs from WT and GF mice on the depleted diet are shown in Figures 3B and 3D.
Previous work from this group demonstrated that the introduction
specific lactobacilli strains could rescue deficits in juvenile
growth in GF Drosophila Melanogaster. As such, the
researchers hypothesized that lactobacilli strains were responsible
for the effects of the microbiota on juvenile growth and on the
somatotropic axis. To test this hypothesis, the researchers
generated two strains of monocolonized mice with one of two strains
of Lactobacillus plantarum, dubbed Lp-WJL and Lp-NIZ02877.
As
Figure 3A,C, and D demonstrates, the monocolonized mouse strains
grow faster (defined by body weight, body length, and femur length)
than GF mice in the standard and depleted diet. Furthermore, Lp-WJL
mice grew faster than Lp-NIZ02877 mice, indicating
that juvenile growth depends on the specific Lactobacillus
plantarum strain. Together, this figure demonstrates that
specific lactobacilli support juvenile growth and protect against
the effects of undernutrition.
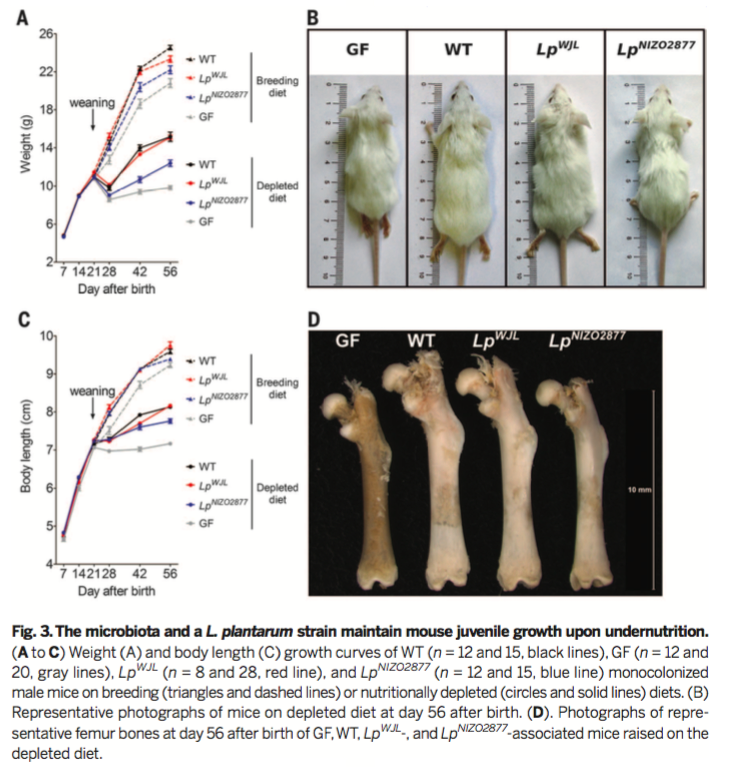
Figure 4
From here, the researchers investigated the effects
of the microbiota on the somatotropic axis during
undernutrition. As in Figure 2, serum levels of GH, IFG-1, and
IGFBP-3 were quantified via ELISA (Figures 4A-C). IGF-1 and
IGFBP-3 levels were significantly reduced in GF mice at 56 days
after birth, which GH was enriched in GF serum at 28 days after
birth. This data indicates reduced somatotropic axis activity in
GF mice during undernutrition.
In Figure 4D-G, WT mice were treated with picropodophyllin (PPP), a selective IGF-1R inhibitor. Dimethyl Sulfoxide (DMSO) was used as a vehicle control. Weight gain, body length, and femur length were all significantly reduced in WT mice in response to PPP, indicating that IGF-1 signaling is necessary for juvenile growth. In the depleted diet group, PPP treatment reduced femur length in WT mice, indicating that IGF-1 signaling is necessary for growth even during undernutrition.
The two monocolonized mice strains were also tested for GH,
IGF-1, and IGFBP-3 in both the standard diet and depleted diet
(Figures 4A-C).
Genomics
Page
Biology Home
Page
© Copyright 2016 Department of Biology, Davidson College, Davidson, NC 28035