This page was produced
as an assignment for an undergraduate course at Davidson College]
Interactions between Mycobacterium
tuberculosis and the immune system by Ben
Buxton
or
"How I survive the proverbial firey furnace" by M.
Tuberculosis
Mycobacterium tuberculosis has
a very puzzling method of pathogenisis. It seems to defy logic by
actually using macrophages, the front line defenses of the human
immune system, as a safe haven for its reproduction. This paper
will discuss the mechanisms of this interaction especially as
they relate to topics covered in Bio 307, Immunology.
The paper is structured as follows:
1. TB disease overview (brief).
2. Recognition and phagocytosis of tuberculi.
3. Pathogen survival in the phagolysosome.
4. Turning the tide - elimination of pathgen.
The spread of tuberculosis (TB) is one of the more present
crises currently facing the human global community. Untreated
cases and multidrug resistant strains often result in death.
Though rates of infection declined with the advent of antibiotics,
TB infection is again on the rise and currently 26 % (2.7 million) of all avoidable
deaths in the world are due to TB (brown.edu, 2000). TB is spread
through inhalation of airborne Mycobacterium
tuberculosis cells
which multiply in macrophages and within the large cystic
tubercles they form - liquified caseous tissue surrounded by
infected macrophages (Bichun et al., 1996). These
inhibit normal lung function and can rupture to spread the
pathogen.
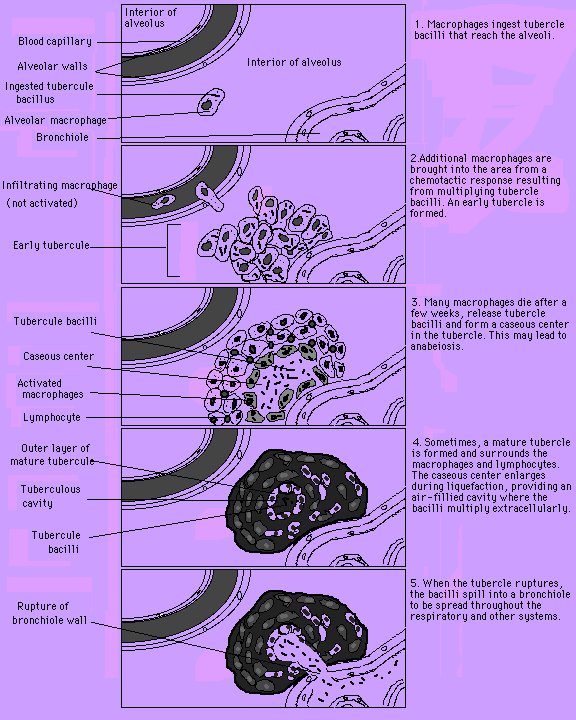
Fig. 1: TB infection of macrophages in the
broader view of tubercle formation in the lung.
(Permission has been requested for the use of this
figure from the site http://virtual.class.uconn.edu/~terry/Spring96/WebTB2/Groups/Group3/Final.html and will be removed if permission is denied.)
Macrophages form a key part of the front line of defense
against invading pathogens in the human immune system. They are
part of the innate immune response and can respond to a broad
range of specific invaders. They act through endocytosis of
pathgens combined with lysosomal action to destroy pathogens.
Endocytosis is mediated by compliment molecules, antibodies and
receptors specific for common bacterial cell wall constituents
which allows action against a variety of pathogens . With a view
towards energy efficiency, macrophages are not normally activated
to full antimicrobial function upon the initial contact with
pathogen and this has dire consequences in the case of
mycobacterial infection (Janeway et al., 1999).
Macrophages must be activated by cytokines released by other
leucocytes order to effectively destroy invading M.
tuberculosis (Banki et al., 1996).
In this early defensive role, alveolar macrophages ingest
inhaled M. tuberculosis cells (as well as other
pathogens) on contact as mediated by compliement receptors CR1, 3
and 4 as well as mannose receptors (MR) (Sinai and Joiner, 1997).
M. Tuberculosis displays lipoglycan molecues on its cell
wall including Lipoarbinomannan (LAM) which, in virulent strains,
includes mannose oligosaccharides at the terminus of the molecule
(Schlesinger et al., 1994). Schlesinger et. al find that
the presence of this mannose terminus inceases attachment of LAM
coated microspheres to macrophages and propose that this is
evidence of an important mechanism of phagocytosis (1994).
Additionally, phagocytosis of mycobcteria is enhanced by addition
of the compliment protein C3 from nonimmune serum, and the serum
protein mannose-binding protein, which recognizes LAM and
lipomannan (Sinai and Joiner, 1997).
LAM, which consists of repeating saccharide units of arabinose
and mannose attached to the mycobacterial membrane through a
phosphatidylinositol moiety, has additionally been directly
implicated in the virulence of M. tuberculosis (Chan et
al. 1991). It functions in suppressing interferon-gamma
induced macrophage activation (through blocking transcriptional
activation of IFN-gamma induced genes), scavenging cytotoxic
oxygen free-radicals and inhibiting protein kinase C activity (Chan
et al., 1991).
The sequence of events occurring to the newly endocytosed M.
tuberculosis containing phagosome is still not fully
understood; contradictory research has been reported (Sturgill-Koszycki
et al. 1994). It seems clear that the normal pathway
leading to the destruction of ingested pathogen must be somehow
altered and the standard explanation for many years has been that
the mycobacterial phagosome does not fuse with lysosomes (Sinai
and Joiner, 1997). Recent research suggests vessicular traffic
with the mycobacterial phagosome does take place, but that the
phagosome has characteristics of an early endosomal compartment
that does not mature or fuse with lysosomes or late endosomal
compartments (Sinai and Joiner, 1997). Macrophage vacuoles
rapidly acidify under normal circumstances, but research has
shown that vacuoles containing mycobacterium are less
acidic than neighboring macrophage lysosomes (Sturgill-Koszycki et
al. 1994). In fact, experiments with closely related M.
avium show a vaculolar pH of 6.3-6.5 for bacterial
phagosomes while normal phagolysosomes show a pH of 5.5 or below
(Sturgill-Koszycki et al. 1994). This same group found
that proton-ATPase vesicles normally fuse with phagosomes soon
after their internalization (rather than being present in the
membrane at the moment of endocytosis), but that Mycobacterium
containing phagosomes prevent fusion with proton-ATPase
containing vesicles while allowing the fusion of other vesicles
including those bearing LAMP-1, normally a phagosomal marker
protein (Sturgill-Koszycki et al. 1994). Subesquent
research showed mycobacterial phagosomes to contain a LAMP level
intermediate between polystyrene bead containing vacuoles and
Legionella phagosomes which nearly lack LAMP (Sinai and Joiner,
1997). In their review of contemporary research, Sinai and Joiner
suggest that the mycobacterial phagosome is somehow arrested in
the early endosome stage and not simply routed to an alternative
pathway of development (1997).
The precise mechanism by which phagosome-lysosome fusion is
prevented has not been elucidated but research suggests that
ammonia produced by M. tuberculosis may play a large
role (Sinai and Joiner, 1997). Since ammonia would rapidly
diffuse out of the lysosome, other weak bases produced by M.
tuberculosis may play a role in preventing normal
acidification - a process necessary for the recruitment of
various coat proteins which may be required to mediate future
fusion events (Sinai and Joiner, 1997)
The immune system mounts a complex response to M.
tuberculosis, and one that is sometimes successful in
stopping the spread of M. tuberculosis. Two cytokines
secreted in response to the pathogen are of particular importance:
IFN-gamma and TNF. IFN-gamma improves H2O2
Production by Macrophages, while TNF can enhance phagocytic and
mycobactericidal activity of macrophages (Barnes et al.,
1990). Investigation has shown that patients with tuberculosis
pleuritis, a form of TB which the body normally recovers from
without administration of therapy, show IFN-gamma and TNF
concentrations 5-30 times higher than blood concentrations (Barnes
et al., 1990). Furthermore, exogenous IFN-gamma was
found to be essential for control of M. tuberculosis in
infected mice of the gko strain with disrupted IFN-gamma genes (Flynn
et al., 1995).
The improved H2O2 production found by
Barnes et al. may prove a moot point as later research
found the action of reactive nitrogen intermediates (RNI) to be
the principle effector mechanism of M. tuberculosis killing,
at least in murine macrophages (Chan et al., 1992).
Effector L-argninine-dependant RNI molecules include NO, NO2
and HNO2 and the pathway has been implicated in
killing activity by macrophages against Toxoplasma gondii,
Chlamydia psittaci, Bacillus Calmette-Guerin, Leishmania donovani
and schistosoma mansoni(Chan et al., 1992).
This group found that murine macrophages activated by IFN-gamma
and LPS or TNF-alpha mounted effective responses to phagocytosed M.
tuberculosis in a manner independant from the action of
reactive oxygen intermediates, suggesting that the production of
RNI is principally responsible for killing and growth inhibiting
ingested pathogen (Chan et al., 1992).
IL-12, secreted by B-cells and macrophages, is another
important cytokine employed against M. tuberculosis. It
induces the production of IFN-gamma from T and NK cells,
enhancing their proliferation in the process, while CD8 T cells
and NK cells are simultaneously down-regulated (Flynn et al.,
1995). Administration of exogenous IL-12 has been shown to
increase resistance of BALB/c mice (which are particularly
susceptible to M. tuberculosis infection), though only
in the presence of IFN-gamma (Flynn et al., 1995). One
is tempted to suggest that IL-12 exerts its effect through the
action of IFN-gamma, but results of IL-12 treatment could not be
replicated by administration of exogenous IFN-gamma (Flynn et
al., 1995). Additionally, one research team found that
exogenous IL-12 did not increase production of IFN-gamma in
infected mice (Flynn et al., 1995). Clearly, we do not
yet have the full picture of IL-12 cytocidal action.
Work Cited:
Banki, A., Jenei, P. M., Richards, G. M. Mycobacterium
tuberculosis and its host cell, the macrophage. 2000.
<http://www.sp.uconn.edu/~terry/Spring96/WebTB2/Groups/Group5/Final.html> Accessed 2000, Apr 13.
Barnes PF; Fong SJ; Brennan PJ; Twomey PE; Mazumder A; Modlin
RL. 1990. Local production of tumor necrosis
factor and IFN-gamma in tuberculous pleuritis. J. Immunol.
145(1): 149-54
Bichun, L.A., Pedrosa, M.M., Trippel, S.J. 1996
Molecular Biology of mycobacterium tuberculosis. <http://www.sp.uconn.edu/~terry/Spring96/WebTB2/Groups/Group14/Final.html> Accessed 2000 Apr 20.
Brown.edu. TB genome. 2000. <http://www.brown.edu/Research/TB-HIV_Lab/tb/tbintro.html>
Accessed 2000 Apr 20.
Chan, J., X-D. Fan, S.W. Hunter, P.j. Brennan, and B.R. Bloom.
1991. Lipoarabinomannan, a possible virulence
factor involved in persistence of Mycobacterium tuberculosis with
macrophages. Infect. Immun. 59: 1755-1761
Chan J; Xing Y; Magliozzo RS; Bloom BR. 1992
Killing of virulent Mycobacterium tuberculosis by reactive
nitrogen intermediates produced by activated murine macrophages. J.
Exp. Med. 175(4): 1111-22
Flynn JL; Goldstein MM; Triebold KJ; Sypek J; Wolf S; Bloom BR
1995. IL-12 increases resistance of BALB/c mice
to Mycobacterium tuberculosis infection. J. Immunol. 155(5):
2515-24
Janeway, Charles A., Travers, Paul, Walport,
Mark, Capra, J. Donald. 1999. Immunobiology: the
immune system in health and disease, 4th Edition. London: Current
Biology. 116-135
Schlesinger, L.S., S.R. Hull, and T.M. Kaufman. 1994.
Binding of the Terminal mannosyl Units of Lipoarabinomannan from
a Virulent Strain of Mycobacterium tuberculosis to Human
Macrophages. Journal of Immunology. 152: 4070-78
Joiner, A.P., Joiner, K.A. 1997. Safe Haven:
the Cell Biology of Nonfusogenic Pathogen Vacuoles. Annu.
Rev. Microbiol. 51: 415-62
Sturgill-Koszycki S; Schlesinger PH; Chakraborty P; Haddix PL;
Collins HL; Fok AK; Allen RD; Gluck SL; Heuser J; Russell DG. 1994.
Lack of acidification in Mycobacterium phagosomes produced by
exclusion of the vesicular proton-ATPase. Science
263(5147): 678-81
![[line]](outdline.gif)
Return
to Davidson College Immunology Home Page


![[line]](outdline.gif)
Direct correspondence to: bebuxton@davidson.edu

![[line]](outdline.gif)
