This web page was produced as an assignment for an
undergraduate course at Davidson College.
G Protein Ras
G protein Ras is a guanine
nucleotide binding protein involved in signal transduction. Ras is
a heterotrimeric protein that associates with the cytoplasmic surface of
the plasma membrane of lymphocytes. It consists of an alpha
subunit, beta subunit, and gamma subunit. The alpha subunit is the
GTP or GDP binding polypeptide which is what binds to the guanine nucleotide
exchange factor (GEF), and the beta/gamma subunit basically functions as
a separate subunit. (Nathanson 1990) When associating with the beta/gamma
subunit, the alpha subunit is in its inactive form when bound to GDP.
When a ligand binds to the receptor of a lymphocyte, the dissociation of
the GDP from the alpha subunit is promoted by guanine nucleotide exchange
factor (GEF) which frees up the binding site allowing GTP to bind.
The alpha subunit then dissociates from the beta/gamma subunit, and phosphorylates
an effetor molecule, a MAP kinase kinase kinase, starting a kinase cascade
by the hydrolysis of GTP to GDP. The alpha subunit is then
inactive and returns to the beta/gamma subunit to repeat the cycle again.
(Sitaramayya 1999 and Zerial 1995))
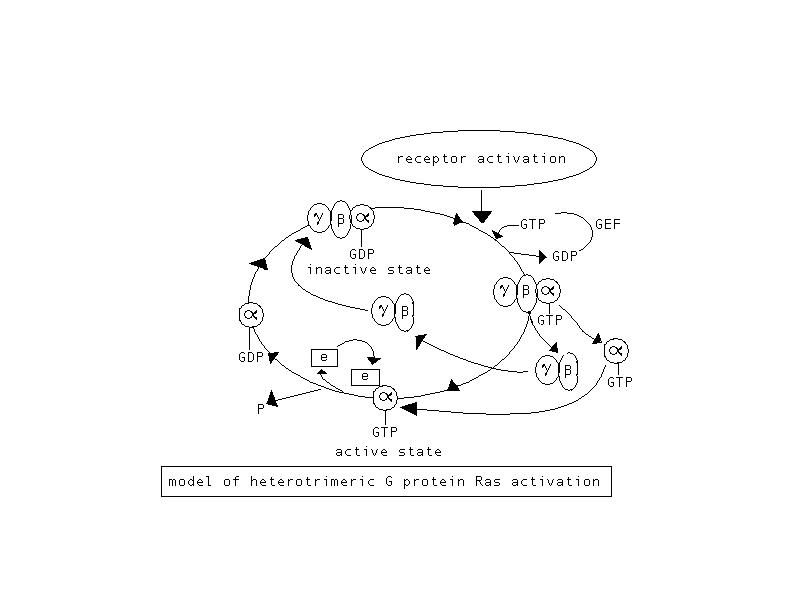
The guanine nucleotide exchange cycle of G protein Ras. Activation
of Ras is caused by receptor activation leading to the removal of GDP then
binding of GTP to the alpha region of Ras. The inactive state of
Ras results from the conversion of GTP to GDP which causes the activation
of an effetor molecule. Adapted from Sitaramayya 1999; figure created
by author of page, Whitney Christian.
Posttranslational Modification of
G Protein Ras
Before
Ras actually takes part in the signal transduction pathway of lymphocytes,
it undergoes posttranslational modification. First, a lipid called
farneyl, which is 15-carbon isoprenoid, is attached to the C-terminal Cysteine
by a process called farnesylation . Then, the last three residues
downstream of this Cys are cleaved proteolytically. Lastly, the C-terminus
is carboxyl methylated. (Sitaramayya 1999)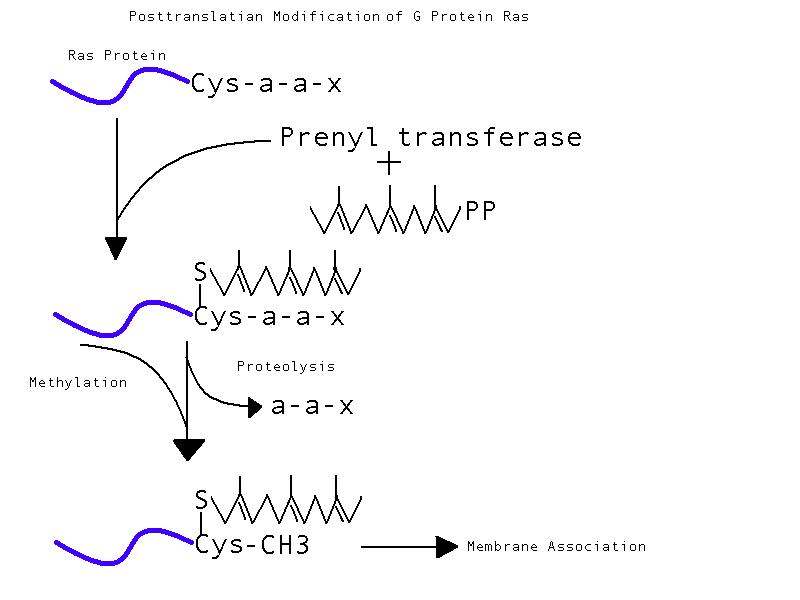
The process of posttranslational modification of
the G protein Ras molecule including farnesylation by prenyl transferase,
cleavage by proteolysis, and carboxyl methylation of Ras. Adapted from
Sitaramayya 1999; figure created by author of page, Whitney Christian.
How does G Protein Ras
participate in the Synthesis of Genes for Proliferation and Differentiation
of Lymphocytes?
In lymphocytes, Ras is involved
in the response to extracellular signals which eventually lead to the activation
of gene synthesis by transcription factors. Once a ligand binds to
the its B-Cell receptor, Blk, Fyn, or Lyn, members of the membrane bound
Src-family kinases, phosphorylate the tyrosine in the immunoreceptor tyrosine-based
activation motifs (ITAMS) of the IgBeta and IgAlpha chains of the B-Cell
receptor creating SH2 domains, binding sites for proteins. Syk then binds
to the newly created SH2 domains of the IgBeta chain and becomes activated
by transphosphorylation, and it is then able to activate SOS, a guanine
nucleotide exchange factor (GEF), with the help of an adaptor protein Grb2.
SOS then removes GDP from Ras and allows GTP to bind, which changes Ras
into its active form allowing the activation of a MAP kinase cascade. (Janeway
et al., 1999)
A MAP kinase kinase kinase
known as Raf is the first kinase to be phosphorylated in the cascade.
Raf in turn phosphorylates a MAP kinase kinase known as Mek which in turn
phosphorylates a MAP kinase, Erk. Erk then activates Elk, a transcription
factor, and Elk enters the nucleus and activates the synthesis of Fos,
another transcription factor and component of the AP-1 transcription factor.
Fos awaits the activation of Jun, the other half of the AP-1 transcription
factor that is activated by the MAP kinase pathway of the B-Cell co-receptor.
Once the Fos and Jun form a heterodimer, the AP-1 transcription factor
is formed, and the synthesis of cell growth genes takes place resulting
in proliferation and differentiation. (Janeway et al, 1999)
Although a different pathway
is followed in T-Cells, the same MAP kinase cascade is ultimately activated.
When T-Cells receptors and their co-receptors are activated by their peptide:MHC
ligands, the union of CD4 and CD45 allows CD45 to activate its tyrosine
phosphatase and remove hindering phosphate groups allowing the activation
of Fyn or Lck, other members of the Src-family protein kinases. They in
turn phosphorylate tyrosine residues on the ITAMS of the CD3epsilon chains
and the zeta chains of the T-Cell receptor. This allows ZAP-70 to
bind by phosphorylation and in turn phosphorylate LAT. LAT recruits
Grb2 which in conjunction with SOS activates Ras which follows the same
MAP kinase cascade as explained earlier. Here too the transcription factor
Fos awaits its counterpart, Jun, which is activated by the MAP kinase pathway
of the T-Cell co-stimulatory molecule CD28. Fos and Jun again join
to form the AP-1 transcription factor, and genes for cell proliferation
and differentiation are synthesized. (Janeway et al., 1999)
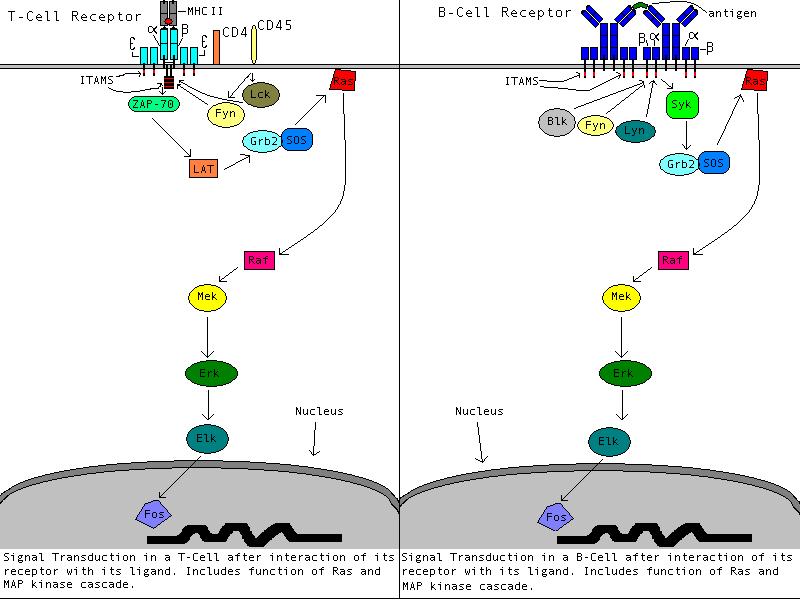
The signal transduction pathways of the T-cell and B-Cell.
Both figures display the function of Ras in their signal transduction pathway.
Adapted from Janeway et al. 1999; figures created by author of page, Whitney
Christian.
References
Alta Vista. Image finder. < http://www.altavista.com>
Accessed 2000 March 1.
Janeway, C.A., Travers, P., Walport, M., Capra J.D.:
Immunobiology:
The Immune System In Health and Disease. 4th Ed. London: Current
Biology Production; 1999. p169-180.
Nathanson, N.M. Ed.: G Proteins and Signal Transduction.
New York: The Rockefeller University Press; 1990. p78-79, 186-187.
Sitaramayya, A. Ed.: Introduction to Cellular Signal
Transduction. Boston: Birkhauser; 1999. p50-51, 253-254, 280-281.
Zerial, M. Ed.: Guidebook to the Small GTPases. Oxford:
oxford University Press; 1995. p3-8, 20-24.
