|
|
Degen and Williams first discovered calnexin in association with MHC class I molecules synthesized by lymphoma cell lines (Bergeron et al, 1994). Calnexin, also referred to as IP90, p88, and p90, is a 88 kDa chaperone protein found in the endoplastmic reticulum. It binds with partially folded immunoglobulin proteins and retains them in the E.R. until they are completely folded (Janeway et al, 1999). Newly synthesized MHC class I alpha chains bind to calnexin until two b microglobulins and possiby a peptide bind to the alpha chain. The heterodimer then dissociates from calnexin and continues on its pathway (Janeway et al, 1999). In addition, calnexin is believed to associate with partial complexes of T cell receptor and B cell membrane immunoglobulin, but not with receptor complexes that are complete.
Figure 1. MHC Class I do not leave the
ER to go to the surface unless they bind proteins.
Courtesy of (Janeway et
al, 1999).
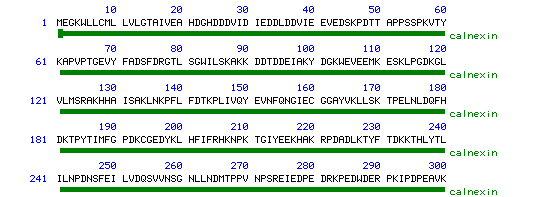
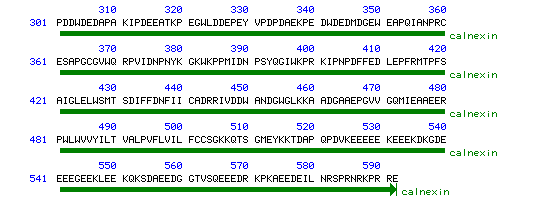
Figure 2. Graphical View of Calnexin Sequence.
Courtesy of <<www.ncbi.nlm.nih.gov>>
Permission to Use Requested
Figure 3. Interactions of a new human
keukocyte antigen (HLA) class I heavy chain
with calnexin during its assmebly . Courtesy
of (Parham, 1996)
Inhibitors of Calnexin Function:
-Bass, J, G Chiu, Y Argon, DF Steiner 1998.
Folding of Insulin Receptor Monomers is Facilitated by the Molecular Chaperones
Calnexin and Calreticulin and Impaired by Rapid Dimerization. Journal
of Cell Biology 141:637-46.
-Bergeron, JJ, MB Brenner, DY Thomas, DB Williams
1994. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum.
Trends in Biochemical Sciences 19:124-128.
-Briquet-Laugier, V, O Ben-Zeev, A White, MH
Doolittle 1999. cld and lec23 are Disparate Mutations
that Affect Maturation of Lipoprotein Lipase in the Endoplasmic Reticulum.
Journal of Lipid Research 40:2044-2058.
-Gahmberg, CG, M Tolvanen. Why mammalian
cell surface proteins are glycoproteins. Trends in Biochemical Sciences
21: 308-311.
-Hammond, C, A Helenius. Folding of VSV
G protein: Sequential Interaction with BiP and Calnexin. Science
266: 456-8
-Janeway, CA, P Travers, M Walport, JD Capra.
Immunobiology:
The Immune System in Health and Disease. New York: Garland Publishers,
1999.
-Monoclonal (Mouse) Anti-Calnexin Antibody (IgG).
<http://www.bioreagents.com/ma3-027h.html>
Accessed
2000 Feb 27.
-Parham, P 1996. Functions for MHC Class
I Carbohydrates Inside and Outside the Cell. Trends in Biochemical
Sciences 21: 428-432.
-Vassilakos, A, M Michalak, MA Lehrman 1998.
Oligosaccharide binding characteristics of the molecular chaperones calnexin
and calreticulin. Biochemistry 37: 3480-90.
Questions, comments - contact: gimooney@davidson.edu