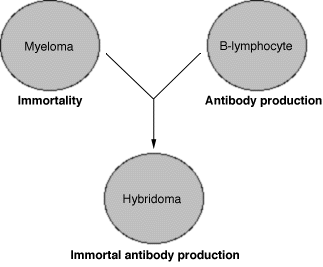
 Figure
2 illustrates the fusion of a myeloma and an antibody-producing cell,
resulting in an immortal, antibody-producing hybridoma.
Figure
2 illustrates the fusion of a myeloma and an antibody-producing cell,
resulting in an immortal, antibody-producing hybridoma.
Monoclonal Antibodies a summary by Kellly E. Westbrook, for Davidson College Biology 304--Molecular Biology
Antibodies, present in the circulation of all vertebrates, are a body's natural defense against foreign substances. When any substance, such as a disease-causing bacteria or virus, is recognized by the body's immune system as an invader--i.e. an antigen--the body produces proteins that bind to that antigen and help destroy it. These proteins, known as antibodies, are very specific and thus bind only to one particular antigen. Once created in response to an antigen, an antibody continues to resist that foreign molecule. This allows our bodies to fight off diseases that previously afflicted us, without getting sick again. For example, most people will only contract chickenpox once in their lifetime, no matter how often they are exposed to the disease. This phenomenon is also what makes vaccinations possible. A vaccine is an injection of killed or weakened virus that stimulates the production of antibodies when introduced into the body.
Antibodies have many useful purposes, in addition to protection against disease. They can be used to diagnose a wide variety of illnesses, as well as to detect the presence of abnormal substances in the blood, such as drugs, viruses, and bacterial products. In order to be used for diagnostic and detection purposes, antibodies that bind specifically to the virus, bacteria or drug of interest are necessary.
When an antigen is introduced into the body, however, a variety of different antibodies are synthesized. The antigen binds to receptors on the surface of B cells, stimulating those cells to produce antibodies specific to the antigen. When seeking out its target, the antibody binds to a site on the antigen known as the epitope. Although each antibody can only bind to one specific epitope, a single antigen may have several effective epitopes, thereby initiating responses from several different B cells. The antibodies produced are referred to as polyclonal antibodies (Campbell 1996).
Until the late 1970s, these polyclonal antibodies, obtained from the blood serum of immunized animals, provided the only source of antibodies for research or treatment of disease. Isolation of specific antibodies was essentially impossible, until 1975, when Georges Kohler and Cesar Milstein discovered how to make monoclonal antibodies from hybridomas (Janeway and Travers 1994).
Hybridomas are hybrid cells that have inherited some characteristics of both parent cells. Kohler and Milstein created hybridomas by fusing malignant myeloma cells with antibody-producing B cells (Figure 2). The myeloma cells have the ability to replicate indefinitely and are clonogenic--they will form clones when grown in vitro. They selected B cells which produced a specific antibody. By fusing these two cell types, they could obtain cells selected for both immortality and production of the specific antibody of interest (Sikora and Smedley).
 Figure
2 illustrates the fusion of a myeloma and an antibody-producing cell,
resulting in an immortal, antibody-producing hybridoma.
Figure
2 illustrates the fusion of a myeloma and an antibody-producing cell,
resulting in an immortal, antibody-producing hybridoma.
The first step in Kohler and Milstein's technique for production of monoclonal antibodies involves immunizing an experimental animal with the antigen of interest. In most of their experiments, Kohler and Milstein injected a mouse with sheep red blood cells. The mouse's body initiates an immune response and begins producing antibodies specific to the antigen. The mouse's spleen is then removed and B cells producing the antibody of interest are isolated. Tumor-producing cells which have been grown in culture are then fused with the B lymphocytes using polyethylene glycol. Only hybridomas resulting from the fusion will survive. The spleen lymphocyte has a limited life span, so any B cells that did not fuse with a myeloma will die in the culture. As well, those cells that lack the antibody-producing aspect of the B cell will not secrete the enzyme HGPRT, which is required for growth in the HAT medium. The hypoxathine-aminopterinthymidine (HAT) medium, on which the cells are grown, inhibits the pathway for nucleotide synthesis. Cells which produce HGPRT can bypass this pathway and continue to grow. By placing the fused cells in a HAT medium, the true hybridomas can be isolated (McKay, Raff, Reichardt 1981).
The isolated hybridoma cells are then screened for specificity to the desired antigen. Because each hybridoma descends from one B cell, it makes copies of only one antibody. The hybridoma that produces the antibody of interest is grown in culture to produce large amounts of monoclonal antibodies, which are then isolated for further use.
Because of their specificity, monoclonal antibodies are more pure than the polyclonal antibodies of conventional techniques and potentially more effective than contemporary drugs in fighting disease. Drugs often attack the body's own cells in addition to the foreign molecule, producing negative side effects such as nausea. Monoclonal antibodies will bind to only the specific target molecule, without any unwanted side effects.
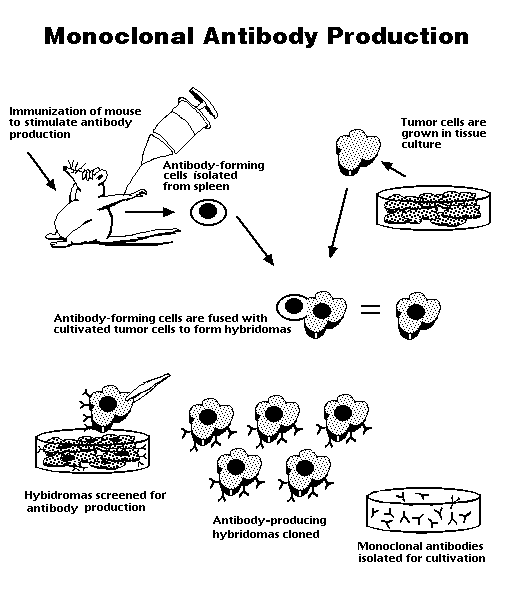
Figure 3 summarizes the production of monoclonal antibodies from a mouse spleen cell.
To supplement your knowledge, there are many current articles you can read on the uses of monoclonal antibodies. You can also learn how to produce your own antibodies by examining the protocol used at the University of Maryland school of medicine. Visual learners can look at an animated antibody, view a short movie (designed by Thomas J. Smith) of an antibody binding to its epitope, or visit a site on "how lymphocytes produce antibody." This additional information may help to augment your understanding of the mechanics and importance of monoclonal antibodies.
Sources
Campbell NA. 1996. Biology, Fourth Edition. Menlo Park, California: The Benjamin/Cummings Publishing Company, Inc. p 860-866.
Janeway CA, Travers P. 1994. Immunobiology. New York: Current Biology Ltd./Garland Publishing Inc. p 23-25.
Lewis GK. 1995. Production of Monoclonal Antibodies. <http.www.som1.ab.umd.edu/Microbiology/protocols/html> Accessed 1998 Feb 18.
McKay, R., Raff, M. C., and Reichardt, L. R., Eds. (1981). Monoclonal Antibodies to Neural Antigens. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory. p 3-9.
Monoclonal Antibody Technology - The Basics. 1989. Washington DC: Biotechnology Industry Organization. <http://www.gene.com/AE/AB/IE/Monoclonal_antibody.html> Accessed 1998 Feb 17.
Sikora K, Smedley HM. Monoclonal Antibodies. <http://www.med.cornell.edu/dept/urology/uo/monoclonal-book04.html> Accessed 1998 Feb 16.