This web page was produced as an assignment for an undergraduate course at Davidson College.
This web page summarizes the paper by Badiola et al.: The Proton-Pump Inhibitor Lansoprazole Enhances Amyloid Beta Production
Introduction:
One pathological hallmark of Alzheimer’s disease (AD) is extracellular amyloid-β (Aβ) peptide deposition in the brain, which leads to energy failure and synaptic dysfunction. These Aβ peptides are caused by the sequential cleavage of the amyloid precursor protein (APP) by two separate proteases: β-site APP-cleaving enzyme 1 and γ-secretase. Recently it has been hypothesized that the toxic component of these Aβ peptides are the soluble fractions, instead of the dense cores of the Aβ plaques as previously thought.
Because few treatments or drugs have been discovered for AD, these researchers used the therapeutic performance mapping system (TPMS) to explore the potential novel interactions between marketed drugs and the biology of AD. TPMS is a system’s biology approach that looks at all known interactions at the molecular level amongst clinical drugs and the body.
This paper does not look at all the results from the TPMS study, but instead looks at one interaction that TPMS suggested: lansoprazole acting as a modulator of the Aβ pathology. Lansoprazole, a proton-pump inhibitor is used to treat peptic ulcer disease. These proton pump inhibitor drugs usually have very few side effects, but because the chronic administration of these drugs is becoming more common, people are wondering about the adverse effects of long-term therapy. This study explores the effects of lansoprazole and other proton pump inhibitor drugs on Aβ production.
Summary of Figures:
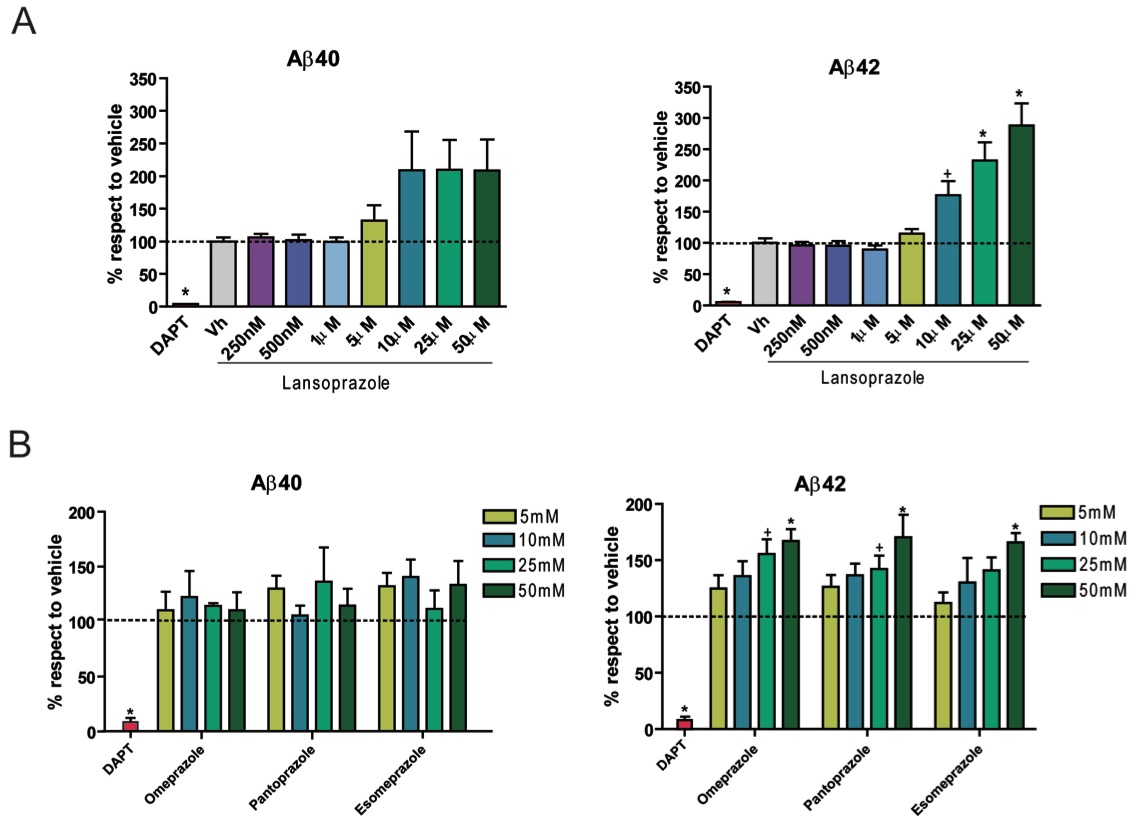
Figure 1: This figure comes from a examination of PS70 Chinese hamster ovary cell lines expressing wild type human APP and presenilin 1. They measure the production of Aβ40 and Aβ42 in the presence of increasing concentrations of lansoprazole and other proton-pump inhibitors, which TPMS also predicted would affect Aβ metabolism. In order to determine the amount of soluble extracellular Aβ products, they compared the amount of the Aβ product in the conditioned media to the amount of Aβ product in the vehicle. The positive control, DAPT, is an inhibitor of the protease that cleaves the APP molecule, which leads to no Aβ production.

A. Extracellular Aβ40 levels had a 2-fold increase with respect to vehicle with concentrations of lansoprazole above 10µM. Extracellular Aβ42 levels increased dose dependently with concentrations between 5µM and 50µM, with a more than 2-fold increase at 25µM dose.
B. Similar treatments with other proton-pump inhibitors showed an increase in Aβ40 levels with concentrations ranging from 5µM to 50µM of the various drugs, but this increase was not homogeneous. However, Aβ42 production increased dose dependently with concentrations ranging from 5µM to 50µM of the various drugs.
Figure 2: γ-secretase, one of the proteases that cleave the APP, can cleave the APP molecule at multiple sites, leading to different Aβ species. Certain small molecule drugs, called γ-secretase modulators and inverse γ-secretase modulators (GSMs and iGSMs), have the capacity to shift where γ-secretase cleaves.
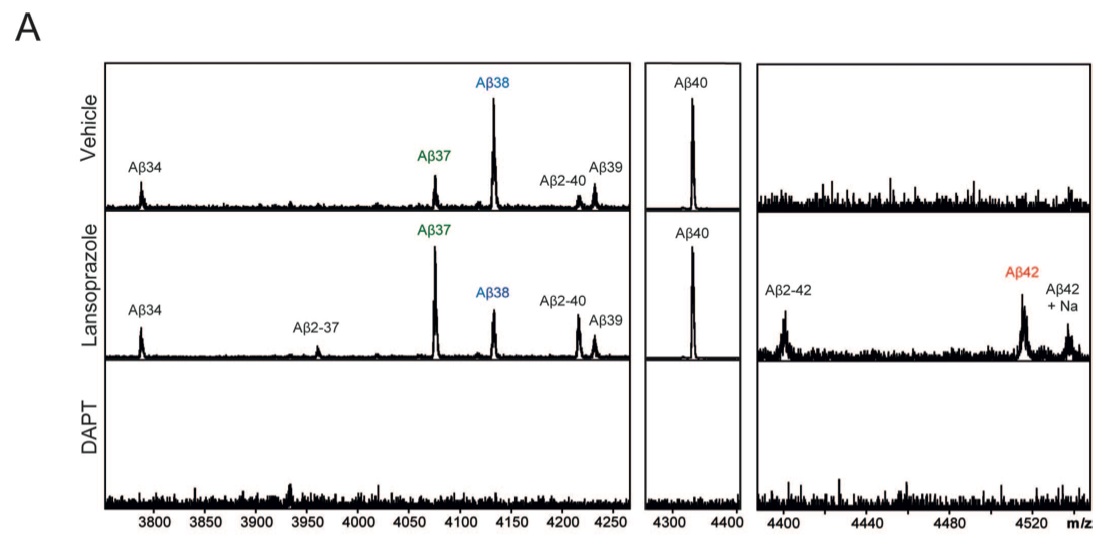
A. Mass spectrometry data shows that in the presence of lansoprazole, the pattern of Aβ species production is shifted; Aβ42 levels increase, while Aβ38 levels decrease. This indicates that lansoprazole may act as an iGSM. Because DAPT, the positive control, inhibits γ-secretase, when it was present no Aβ peaks are seen.
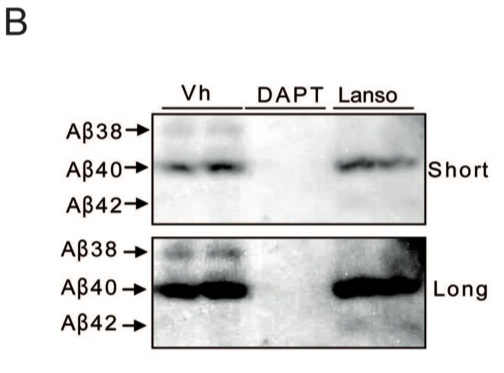
B. A western blot was used to further confirm the increase in Aβ42 levels and decrease in Aβ38 levels in the presence of lansoprazole. Both short and long exposures are shown to emphasize the differences. When looking at the short exposure, it appears as if the Aβ38 blot is gone when it was exposed to lansoprazole. In the long exposure panel, Aβ42 is only visible when it was exposed to lansoprazole.
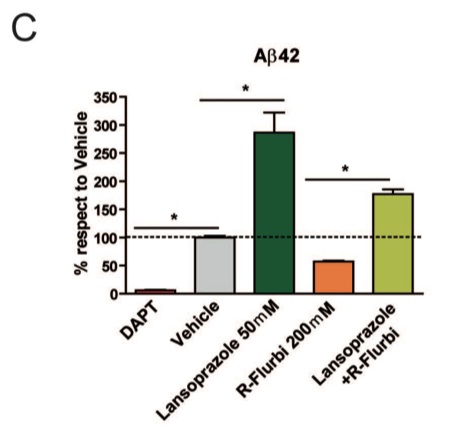
C. R-flurbiprofen, a non-steroidal anti-inflammatory drug and GSM, which has been explored as a therapeutic drug for AD, lowers Aβ42 levels. The researchers wanted to determine if lansoprazole could counterbalance the effects of R-flurbiprofen. DAPT, the positive control decreased the Aβ42 levels to close to zero as expected. When only R-flurbiprofen was added, Aβ42 levels decreased, and when only lansoprazole was added, Aβ42 levels increased, as expected. However, when lansoprazole and R-flurbiprofen were added, the Aβ42 levels were significantly lower than the levels with just Lansoprazole and significantly higher than the levels with just R-flurbiprofen, indicating that Lansoprazole can counter the effects of R-flubiprofen.
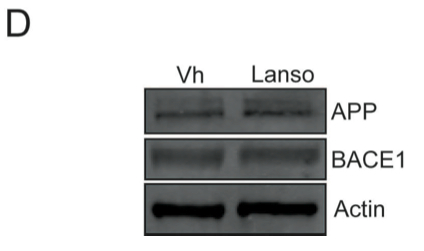
D. In order to understand the reason for the increase in Aβ40 and Aβ37 levels in the presence of lansoprazole, they looked at the effects of lansoprazole on some of the proteins involved in the APP processing pathway. The researchers examined the effects of lansoprazole on APP, BACE1 (the first enzyme to cleave APP), and actin (the negative control). The western blot shows no difference in the protein levels between treated and untreated cells.
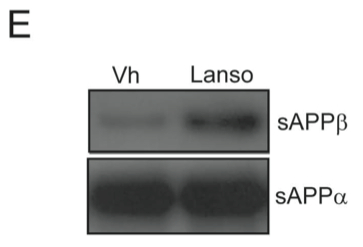
E. They also analyzed the amounts of sAPPβ and sAPPα, the initial products when APP is cleaved by BACE1 to determine if lansoprazole enhances BACE1 activity. These molecules, after further cleaving, become Aβ40 and Aβ37. The western blot indicates that there is an increase in sAPPβ levels in the presence of lansoprazole.
In summary, Figure 2 illuminates some of the ways lansoprazole interacts with the proteins that cleave APP into the various smaller peptide chains. From this data, it appears as lansoprazole modulates γ-secretase, acting as a iGSM, and increases BACE1 activity.
Figure 3: These new findings about the way lansoprazole interacts with the enzymes that cleave APP can impact AD research because lansoprazole can pass the blood brain barrier, and because of this, it has the potential to affect the morphology of the brain tissue. Therefore, using mouse models, the researchers measured the levels of soluble Aβ40 and Aβ42 in the brain after intraperitoneal administration of lansoprazole to wild type and AD triple-transgenic mice. AD triple-transgenic mice overexpress human tau and APP, with presenilin 1 expressing in the background. Both wild type and AD triple-transgenic mice were administered a 100 mg/kg/day dose of lansoprazole for 5 days. Another group of AD triple-transgenic mice were also administered a 20 mg/kg/day dose.
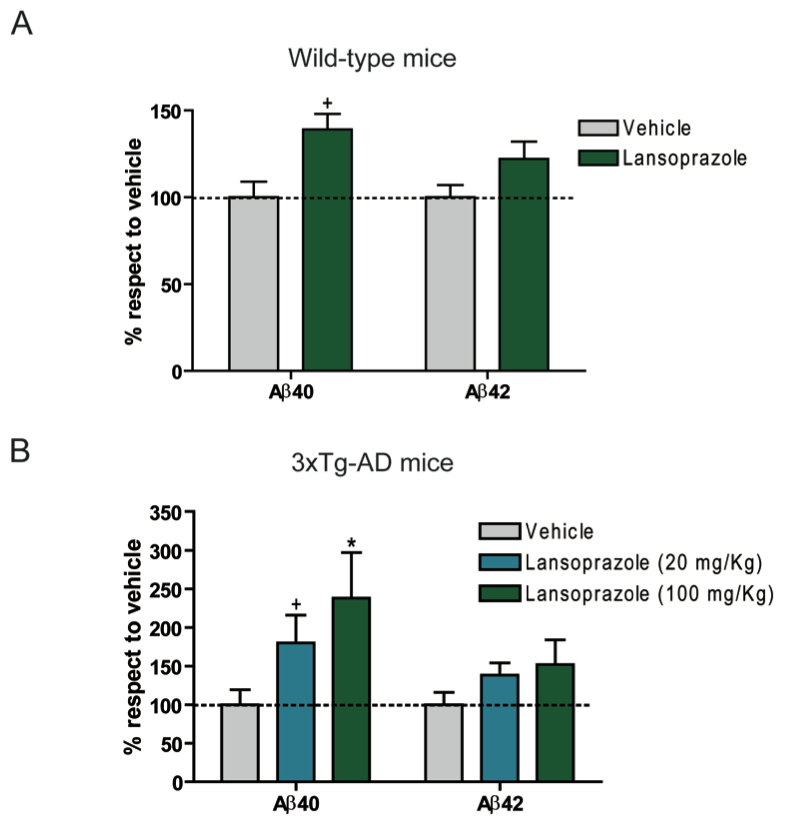
A. After the administration of lansoprazole, the wild type mice showed an increase in soluble Aβ40 and Aβ42 levels in the brain, but only the increase in Aβ40 was significant.
B. Soluble Aβ levels in AD triple-transgenic mice increase in a dose dependent manner. There is a significant increase in soluble Aβ40 levels with a 20 mg/kg dose of lansoprazole and an even greater increase with a 100 mg/kg dose of lansoprazole. This increase was greater in the AD triple-transgenic mice than in the wild type mice. A dose dependent increase in Aβ42 was also seen, but it was not significant.
Figure 4:
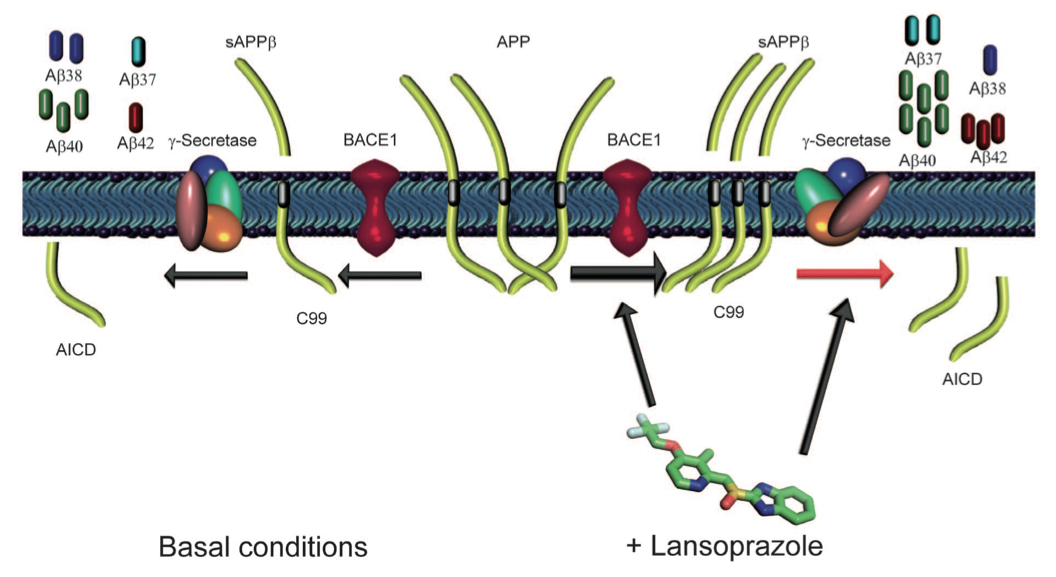
This schematic diagram summarizes what was seen in the experimentation, showing a hypothetical mechanism for how lansoprazole affects Aβ production. The left half of the diagram shows the basal cleaving patterns and the right half of the diagram shows the cleaving patters in the presence of lansoprazole. The center of the diagram shows the APP structures (three long green tubes). In the absence of lansoprazole (moving toward the left), BACE1 cleaves the APP protein into sAAPβ and C99 peptides. Then, γ-secretase cleaves C99 into AICD, and sAPPβ into Aβ38, Aβ37, Aβ40 and Aβ42. In the presense of lansoprazole, the drug enhances BACE1, making it more active so that it cleaves more APP into sAAβ and C99. Lansoprazole also changes the shape of γ-secretase, shifting the cleavage sites so that it produces more Aβ37, Aβ40, and Aβ42, but less Aβ38.
Conclusions:
Badiola et al. used a system’s biology approach to determine potential interactions between marketed drugs and the biological mechanisms of Alzheimer’s disease. This systems biology approach indicated that lansoprazole could modulate the amyloid-β pathology process, an interaction, which was unknown before this, but could be tested.
In order to investigate the effects of lansoprazole, the researchers only looked at the soluble Aβ forms. I think this is a weakness in the study. Although they indicate that the hypothesis that the toxic part is in the soluble portion is gaining support, it has not been shown conclusively. Several studies have supported the hypothesis that the Aβ plaques are harmful to the brain.
Another weakness in the presentation of the data is the lack of loading controls on the western blots seen in Figure 2. In figure 2.B, we are supposed to see a decrease in Aβ38 and an increase in Aβ42, but these changes are very slight, and without a loading control it is difficult to determine the significance of these differences.
Overall though, this paper showed how the drug lansoprazole, in doses comparable to that which is given to humans to treat various diseases, can cause changes to the amyloid-β metabolism process, which could be a factor in Alzheimer’s disease. They confirm these results both in vitro and in vivo. By using a system's biology approach initally, the researchers were able to hone in on one particular drug and then experimentally determine the impacts it could have on the pathology of Alzheimer's disease, tentatively confirming the hypothesis that lansoprazole affects Aβ production. More clinical research should now be done to determine how the administration, especially for long-term therapy, of lansoprazole affects Alzheimer's disease.
Acronym Glossary:
References:
Genomics Page
Biology Home Page
Email Questions or Comments to Becca Evans.
© Copyright 2013 Department of Biology, Davidson College, Davidson, NC 28035