This web page was produced as an assignment for an undergraduate course at Davidson College.
The paper investigated restoring normal skeletal muscle function impaired by deliberately denervated peripheral nerve in mice using a novel combination of optogenetics and regenerative medicine. Earlier approaches to restore function through nerve regulation by electricity and replacement of motor neurons with stem cells have had success but have critical limitations. Electrical stimulation can be used to regulate muscle function in nerve-damaged tissue but has the disadvantage of indiscriminate nerve stimulation causing pain in subjects. Embryonic stem cells (ESCs) can be engrafted in the central nervous system to compensate for lost motor neurons but have issues integrating into the central nervous system limiting restoration of normal muscle function.
In attempt to develop a new strategy to restore normal muscle function researchers made use of a synthesis of optogenetics, the field of making neurons sensitive to light through genetics, and regenerative medicine, the restoration of normal tissue function through replacement with lab grown cells or organs. Motor neurons that express light-sensitive ion channel channelrhodopsin-2 (ChR2) can be optically stimulated. Researchers engrafted ESC derived from nerve cells and expressing ChR2 into denervated peripheral nerves in mice. The objectives of this procedure as to determine if the ChR2 expressing ECS would restore control and motor function without affecting either endogenous motor signals or afferent sensory axons.
Restoring nerve control with the novel approach taken by the researchers is an interesting pursuit and it is worthwhile to continue the research detailed in the paper. The background of the authors provides a concise summary of the limitations of current approaches thus necessitating a new approach they hope to provide. The paper was not difficult to read and it was rather easy to follow the procedures the researcher conducted. However the figures presented by the paper were dense and thus their meanings were not clear. Figure 2 panel F was difficult to interpret and should have had additional explanation.

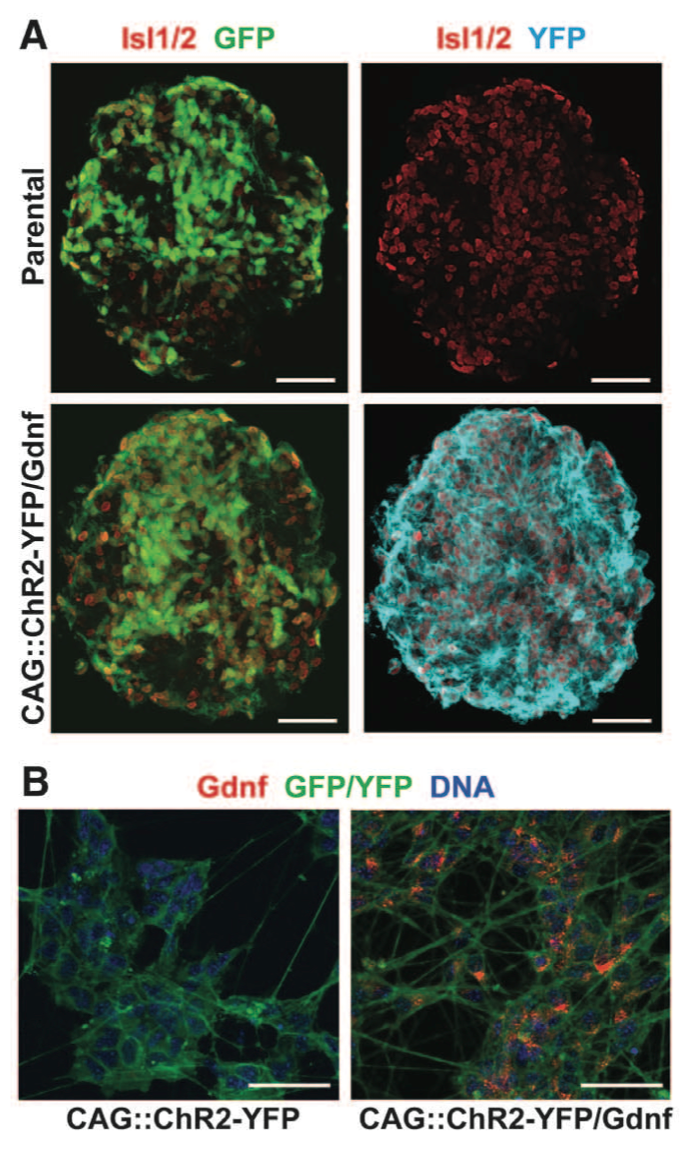
Figure 1. Panel A & B Panel A is a comparison of parental ESCs compared with transgenic CAG::ChR2-YFP/Gdnf ESCs for fluorescent proteins GFP and YFP. The mice ESC clonal cell lines carried Hb9::CD14-IRES-GFP which expresses GFP and appears in both the parental and transfected ESCs. The parental ESCs were transfected with CAG::ChR2- YFP which produces YFP and is reflected in the absence of YFP in the parental line. Panel B is a comparison of both CAG::ChR2- YFP against CAG::ChR2- YFP/Gdnf. Gdnf expression is observed only in the YFP/Gdnf variant.
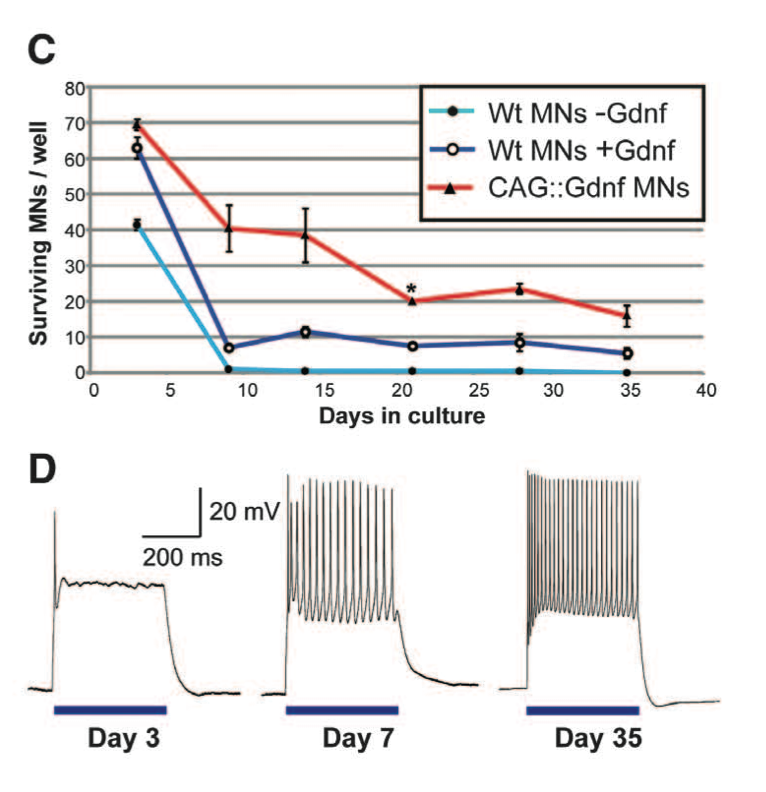
Figure 1. Panel C & D Panel C compares the survival of wild type motor neurons, wild type with Gdnf, and CAG::ChR2-YFP/Gdnf in a culture over a 35 day period. 200 cells of one cell type were plated per well. Both wild type cells with and without Gdnf had low survival rates compared to CAG::Gdnf MNs. Panel D portrays optical stimulation of ESCs with CAG::ChR2-YFP/Gdnf to point out the maturation of the motor neurons over a period of 35 days. Motor neurons had neuron activity patterns from optical stimulation similar to motor neuron patterns caused by electrical stimulation. Figure gives background on the ECS used by detailing the success of transfecting the ECS with CAG::ChR2-YFP/Gdnf both in terms of survivability and neuron stimulation.
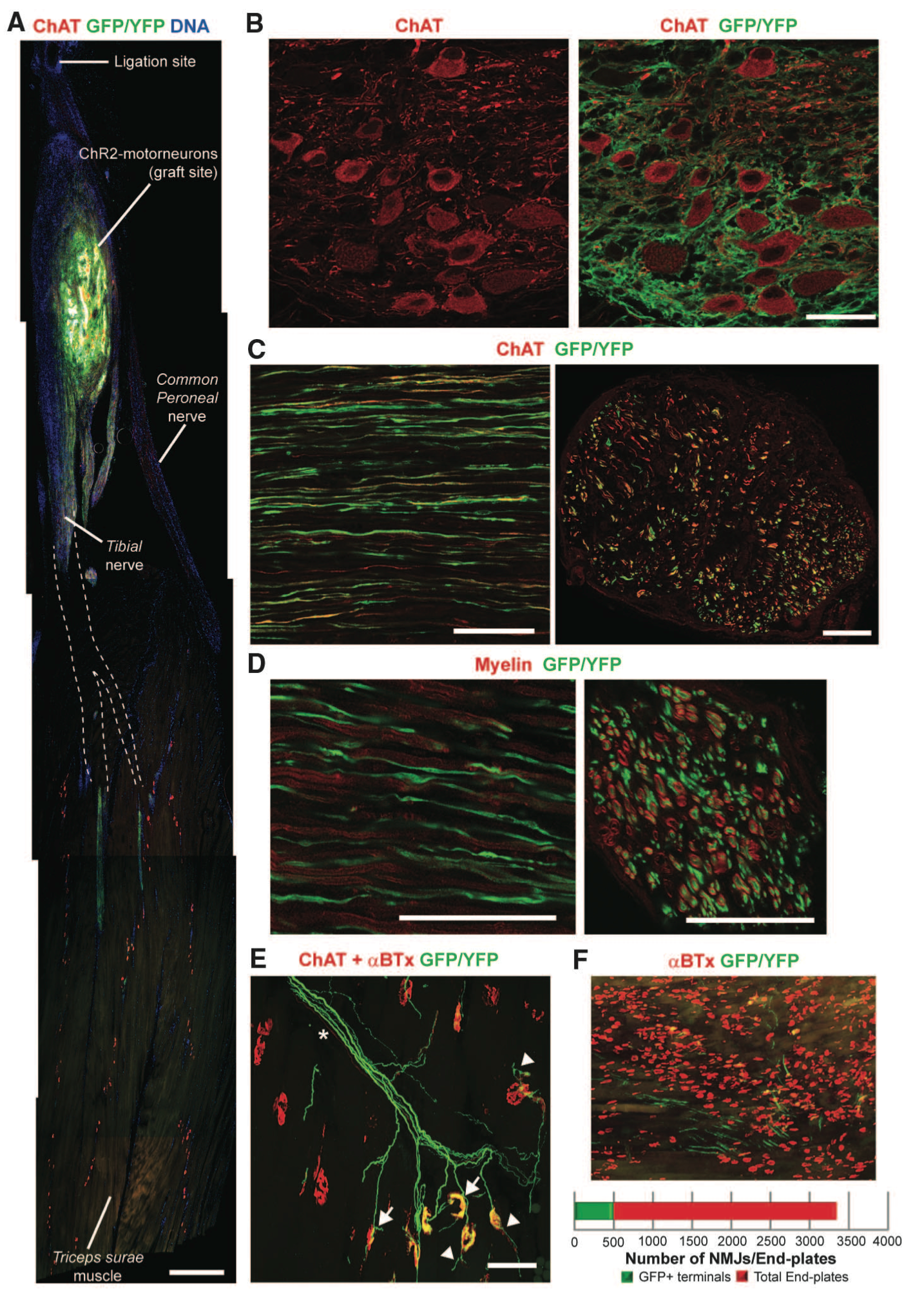
Figure 2. Panel A shows the graft site location of the ChR2 motor neurons onto the sciatic nerve and axon projection. Panel B identifies motor neurons immunolabeled using choline acetyl transferase (ChAT). Grafted ChR2 motor neurons are identified by their GFP and YFP expression. Panel C is a longitudinal version of Panel B intended to show the location of ChR2 motor neurons and endogenous axons. Motor neurons express GFP/YFP while endogenous axons are illustrated in red through immunolabeling with ChAT. Panel D identifies myelination of ChR2 motor neurons through ChAt and GFP/YFP. Panel E shows the ChR2 motor neurons innervating at neuromuscular junctions in the triceps surae (TS) muscle in mice. Panel F shows an illustration of the proportion of neuromuscular junction innervated by ChR2 motor neurons. The purpose of figure 2 is to show the engraftment and integration of the ChR2 motor neurons.
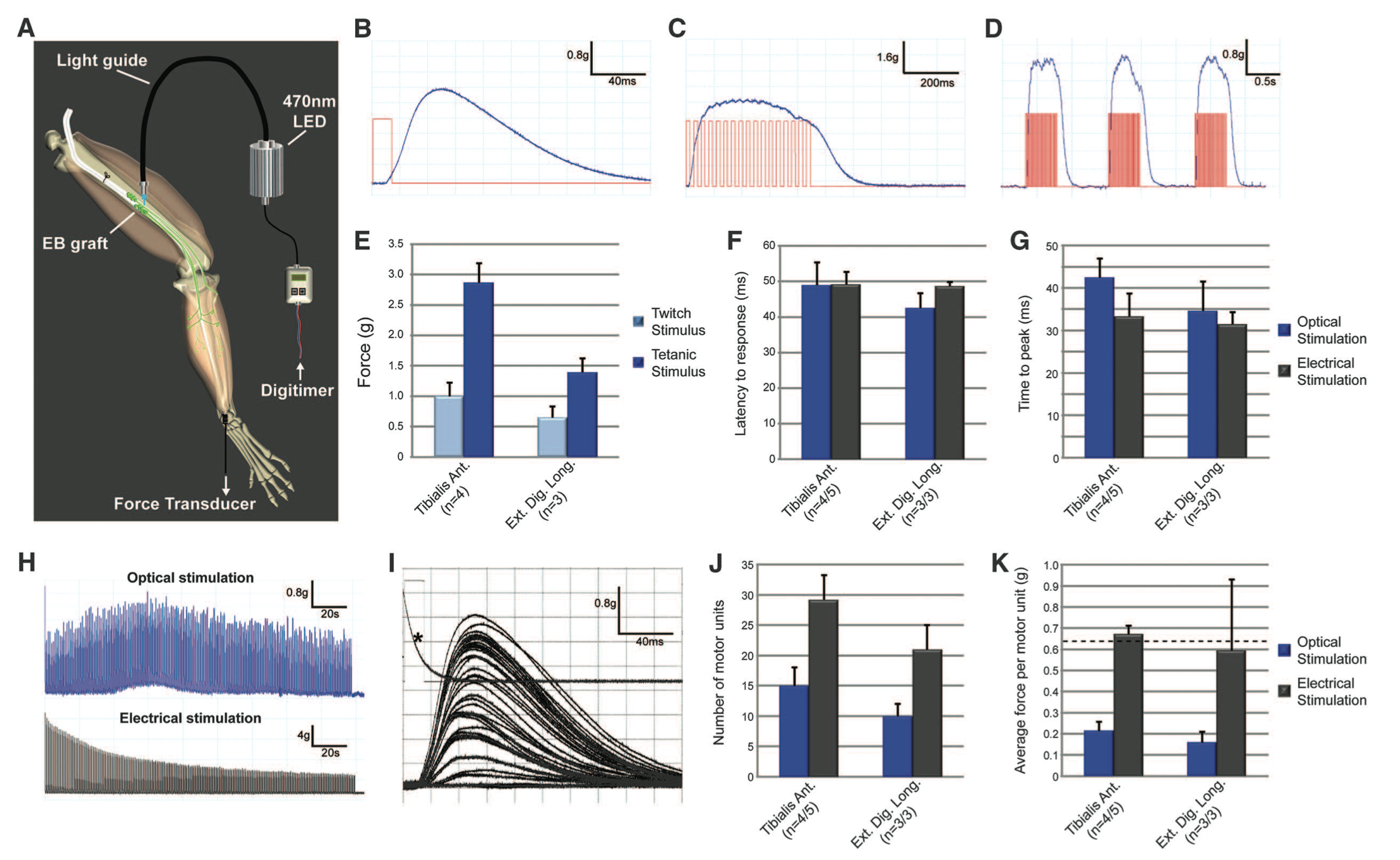
Figure 3. Figure3. Figure 3 demonstrates optical stimulation of the engrafted ChR2 motor neurons in vivo by quantifying twitch, tetanic, and repetitive tetanic contraction traces in the tibialis anterior and extensor digitorum longus (EDL) muscles. Contraction force was quantified for both the endogenous axons and ChR2 motor neurons using electrical stimulation. Optical stimulation matched contraction force obtained by electrical stimulation.
Bryson, JB et, al. 2014. Optical Control of Muscle Function by Transplantation of Stem Cell-Derived Motor Neurons in Mice. Science 344: 94 - 97
Andrew Liu's Homepage
Genomics Page
Biology Home Page
© Copyright 2014 Department of Biology, Davidson College, Davidson, NC 28035