
Scanning
electron micrograph of various bacteria in a human fecal sample.
The human gut contains a wide variety of microbes that are strongly
linked to human health
and
disease. The establishment and function of the gut microbiota during
development is an
increasingly
popular subject of research. Image courtesy of Alimentarium.
Permissions pending.
Summary
Schwarzer
et al. (2016) sought to examine
the possible influence of the gut microbiota on postnatal growth under
normal conditions and during chronic undernutrition. Overall, they
demonstrate that gut microbiota contribute to and are important for
normal juvenile growth in mice, as measured primarily by weight gain and
longitudinal growth. The microbiota exert their effect on systemic
growth via interaction with the somatotropic axis, a network that drives
systemic postnatal growth in mammals. Furthermore, a specific Lactobacillus
plantarum strain was identified as sufficient in promoting growth
and “buffering” growth-stunting effects of chronic undernutrition.
This bacterial strain promotes juvenile growth via action on
the somatotropic axis. These findings have important implications for
public health, particularly in third-world countries, because microbial
interventions of specific bacterial strains could be used
therapeutically to combat the adverse growth effects of chronic
undernutrition.
Opinion
I find this paper to be relevant and compelling overall. They asked a novel question about how the gut microbiota influences juvenile growth under different nutritional conditions, which is innately interesting in light of increasing interest in the microbiome and potentially applicable in the context of public health. In terms of the way the paper was laid out, I particularly liked they way they drew clear links between the gut microbiota, the somatotropic axis, and postnatal growth. The general flow of the paper was logical, and I was able to understand what experiments they performed and why they performed them without having to do additional research on my own. I also appreciated that they provided multiple pieces of evidence to support one general conclusion. For example, they drew conclusions about growth by assessing body weight, length, and multiple bone growth parameters, and they drew conclusions about somatotropic axis activity based on data from multiple components involved in the network such as circulating levels of GH, IGF-1, and IGFBP-3 and phosphorylation of Akt.
There were also a few aspects of this paper related to data visualization that could stand to be improved. The first is that, in a few cases, the researchers expected the reader to draw quantitative conclusions, but provided pictures as evidence rather than quantitative representations of the data. This discrepancy was primarily an issue with the bone growth parameter data, where images of femurs were often provided in place of graphs. Though a difference in femur length or density is somewhat visible in these images, the degree and significance of that difference across multiple individuals would be much more convincing if it was presented quantitatively, such as in a graph. The figure organization of this paper could also be improved. The reader sometimes had to jump from figure to figure as they read the text, rather than having the figures flow in a logical manner with the text. Occasionally, the researchers would even make comparisons between components of separate figures. For example, the reader is asked to compare GH and IGF-1 levels between breeding and chronic undernourishment conditions by comparing data in Figure 2 to that in Figure 4. Providing additional figures or panels that directly depicted these comparisons would aid the reader in drawing the same conclusions as the researchers, thus strengthening the researchers' overall argument.
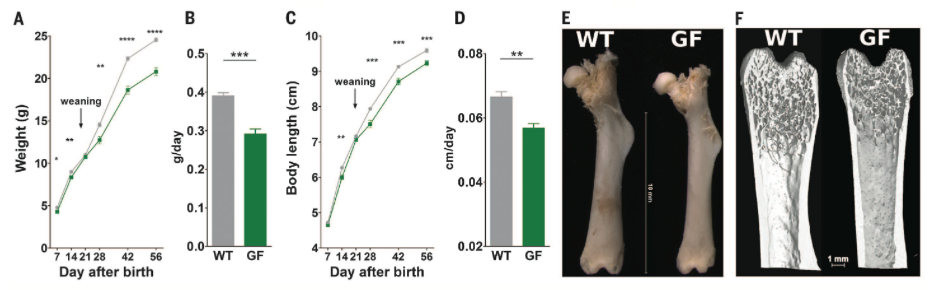
Figure 1. Demonstrates the effects of the microbiota on juvenile growth by comparing growth of wild type (WT) and germ free (GF) mice. Body weight and body length were measured and growth rates were calculated. Femur length was measured using a calliper and a high resolution nanotom device was used to assess 3D microarchitecture such as cortical thickness. Body weight and length (A and C, respectively) over time as well as their average rates of increase (B and D, respectively) were lower in GF mice as compared to WT. Femur growth parameters (E and F) were also decreased in GF mice.
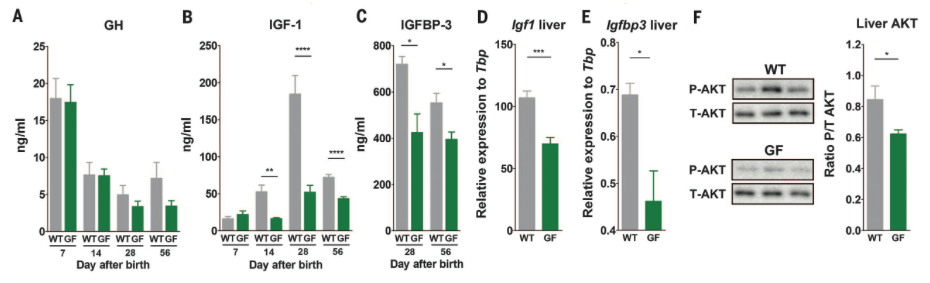
Figure 2. Demonstrates effects of the microbiota on activity of the somatotropic axis by comparing various features of the somatotropic axis between WT and GF mice. Serum levels of GH, IGF-1, and IGFBP-3 (A, B, and C, respectively) were measured using the ELISA method. GH levels similarly decreased over time in WT and GF mice, and levels of both IGF-1 and IGFBP-3 were reduced in GF mice. Expression of Igf1 and Igfbp3 (D and E, respectively) in liver was quantified via quantitative RT-PCR, and expression of both genes was reduced in GF animals at 28 days after birth. Western blot was used to assess phosphorylation of Akt at Ser 473, which marks IGF-1R activity, in liver (F) and phosphorylation was reduced in GF compared to WT mice.
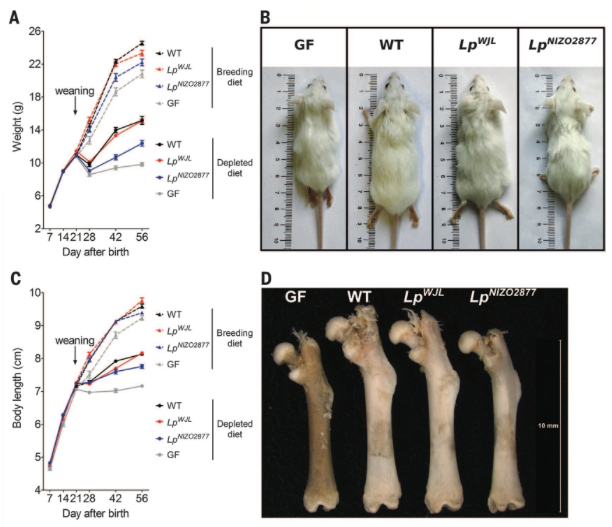
Figure 3. Shows contribution of microbiota to postnatal growth under conditions of chronic undernourishment. WT, GF, or mice with only one of two specific strains of Lactobacillus plantarum (WJL and NIZO2877) were fed normal breeding diets or depleted diets. The same methods employed in Figure 1 were used to assess juvenile growth. All chronically undernourished mice lost weight and slowed rate of body length increase after weaning (A and C). For mice on both breeding and depleted diets, WJL and NIZO2877 mice grew better than GF mice, but WJL mice grew better than NIZO2877 mice, growing nearly as well as WT mice (A, B, C, and D). Taken together, these findings indicate that specific bacterial strains can "buffer" the growth-stunting effects of chronic undernourishment.
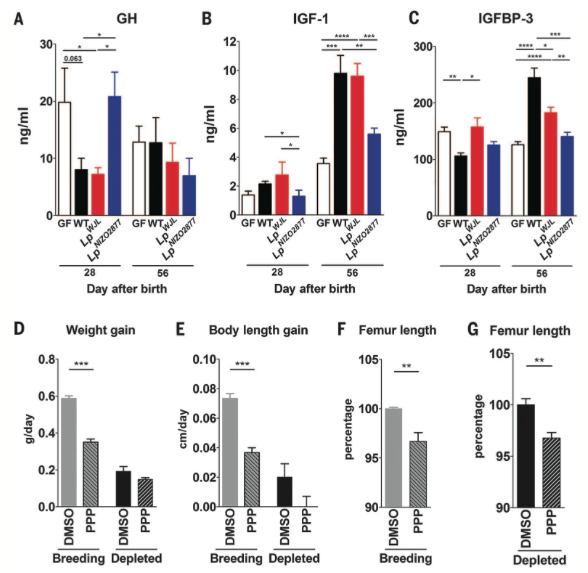
Figure
4. Shows effects of gut microbiota on activity of somatotropic
axis during chronic undernutrition. Serum levels of GH, IGF-1, and
IGFBP-3 (A, B, and C, respectively) were measured via ELISA in GF, WT,
WJL, and NIZO2877 mice at two time points. Overall, somatotropic
activity is reduced in chronically undernourished GF mice. During chronic
undernourishment, the WJL but not the NIZO2877 bacterial strain was
sufficient to at least partially increase somatotropic activity,
approaching that of WT mice. Rates of weight gain (D) and body length
(E) as well as femur length (F and G) were measured to compare growth of
WT mice injected (twice daily for ten days) with DMSO (control) or PPP
(inhibits IGF-1R and thus somatotropic activity). Overall, injection with
PPP reduced the growth of WT animals, demonstrating that somatotropic
activity is required for postnatal growth.
Reference
Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, Martino ME, Balmand S, Hudcovic T, Heddi A, Rieusset J,
Kozakova H, Vidal H, Leulier F. 2016. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition.
Science.
351(6275):854-857.
Genomics
Page
Biology Home Page
Email Questions or Comments: moshannon@davidson.edu
©
Copyright 2016 Department of Biology, Davidson College,
Davidson, NC 28035