If you wanted to know whether a particular promoter were activated or not, you'd like to be able to determine its activity without having to measure mRNA levels which is very difficult. You'd like to have an easy way to see that a promoter is activated. Or perhaps you would like to be able to watch your favorite protein as it traveled around a cell performing its cellular role. One protein has been very successful at both of these molecular methods - Green Flourescent Protein (GFP; figure 1).


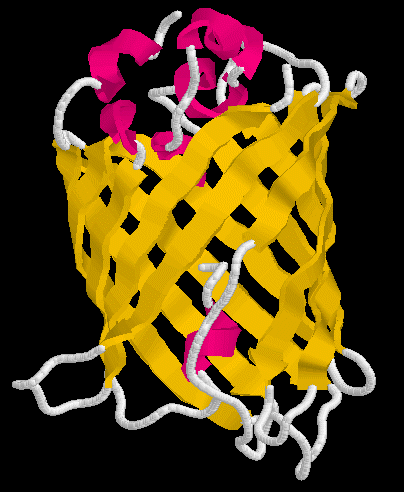
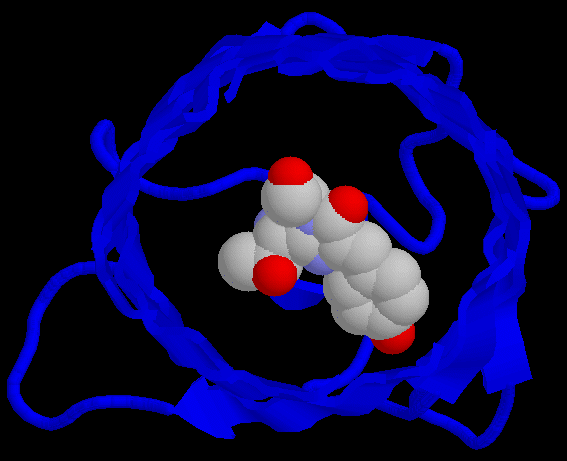
Figure 1. Structure of GFP. Figure on left shows the alpha helices in red and beta sheets in yellow. The figure on the right shows the barrel shape of GFP with the chromophore amino acids 65-67 highlighted in the center of the barrel.
GFP was the first of many flourescent proteins to be used as a reporter protein. When excited with a blue light, it will flouresce green. It is very stable, can function when added to either end of a protein of interest, and does not fade easily. GFP has become so popular that mutant alleles have been created that produce different colors of light. Furthermore, a naturally occurring red flourescent protein has been cloned from a different organism. In short, GFP is very popular and it has many uses. An interactive chime figure appears below.
This is a monomer of GFP. You are looking into the barrel of the protein.
© Copyright 2002 Department of Biology, Davidson College, Davidson, NC 28036
Send comments, questions, and suggestions to: macampbell@davidson.edu