The present study indicates that PABA selectively interacts with two cytosolic proteins, p51 and p38, in mammalian nervous tissue. A protein of 44 kDa also appeared in PABA high salt fractions but showed staining characteristics similar to a 41 kDa protein common to control fractions. The intense staining and consistency throughout flow through and gradient fractions of both experimental and control gels suggests that the two proteins are the same. Due to the extreme protein sensitivity of silver staining, it is supposed that the 44 kDa and 41 kDa proteins are keratin (Deutscher, 1990).
Keratins are a class of filament proteins of 40 kDa to 70 kDa and produced by epithelial cells, found in skin, hair, nails and many other tissues (Gupta et al., 1992). The proteins unique to the PABA fraction are assumed not to be keratin because the PABA and control gels were stained together, thus exposing them to identical environments. Furthermore, the 44 kDa protein band appears of consistent intensity and size throughout the gradient fractions for both PABA and control gels while the 51 kDa and 38 kDa proteins increase in intensity as the gradient salt concentration increases. The increasing band intensity is indicative of ligand recognition because the higher salt concentration decreases ionic interactions thus disabling the ability of PABA to effectively bind particular proteins causing them to elute from the column. Finally, by simultaneously staining the gels, it also limits the variable of developing time.
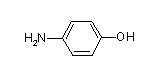
Though I have not fully characterized the proteins isolated from the PABA column, previous studies provide evidence suggestive of their identity. PABA is a substrate common to many enzymes involved in biosynthetic pathways. 4-aminobenzoate hydroxylase is a FAD-dependent monooxygensase that catalyzes the decarboxylative hydroxylation of PABA to form 4-hydroxyaniline (Fig. 19) used in the synthesis of phenylalanine, tyrosine, and tryptophan. 4-aminobenzoate hydroxylase carries out the reaction in the presence of NAD(P)H and O2 (GenomeNet "1.14.13.27", 1999).

Another possibility is the enzyme 7,8-dihydropteroate synthase (DHPS) that acts in the folate biosynthetic pathway. DHPS catalyzes a reaction between 2-amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine diphosphate and PABA that produces pyrophosphate and dihydropteroate (Fig. 20) (GenomeNet "2.5.1.15", 1999). Though higher eukaryotes cannot synthesize folic acid de novo and thus do not produce DHPS, the structural similarity of the enzymatic product, dihydropteroate, to AIT-082 is not worthy. Both compounds have a PABA portion linked to a fused bi-heterocyclic system. There may be an enzymatic homologue in higher eukaryotes that interacts with AIT-082. DHPS like 4-aminobenzoate hydroxylase acts in pathways that produce compounds and intermediates associated with Alzheimerís disease and other neurodegenerative disorders. Folate deficiencies have been cited in Alzheimerís and neurological disorders associated with AIDS and Down Syndrome (Regland and Gottfries, 1992).
Heme oxygenase 2 is a 35 kDa carbon monoxide producing enzyme found predominately in mammalian brain and testes tissues (Rotenberg and Maines, 1991; Rublevskaya and Maines, 1994). Heme oxygenase 2 has shown in situ stimulation of soluble guanylyl cyclase in neurotransmission and vasorelaxation (Serfass and Burstyn, 1998). Though preceding research has not linked PABA to heme oxygenase 2, the enzymeís involvement in the mechanism of AIT-082 makes it an attractive candidate. A final possibility also exits that the isolated proteins are in fact unique and have not been characterized at all.
The proteins PABA binds provides suggestions as to its role in AIT-082. A major attribute of AIT-082 is its ability to cross the blood brain barrier (BBB). PABA by itself shows little ability to cross the BBB (Rapoport, 1976; Elo et al., 1982). Hypoxanthine, the other half of AIT-082, unlike PABA shows receptor-mediated transport across the BBB (Spector, 1987). The ability of hypoxanthine to cross the BBB and PABAís relatively poor transfer across the BBB suggests that PABAís role in AIT-082 is not one of an absorption enhancer. Once inside the brain AIT-082 demonstrates a biological effect different than an aggregate of the effects of hypoxanthine and PABA acting independently of one another (Glasky, 1992). The novel, non-additive effect invoked by AIT-082 suggests that the bridge between the hypoxanthine and PABA is maintained. The maintenance of the tether does not preclude the possibility that PABA interacts with either a DHPS homologue or 4-aminobenzoate hydroxylase. The enzymatic product of 4-aminobenzoate hydroxylase, 4-hydroxyaniline (Fig. 19), is merely a change in the acid functionality of PABA to an alcohol functionality. Since hypoxanthine is bridged to PABA at the amino end opposite the alcohol functionality, hypoxanthine may be able to situate itself so that it does not interfere with that reaction. In the case of a DHPS homologue, the product dihydropteroate (Fig. 20) from a structural standpoint closely resembles AIT-082. Though the action of a DHPS homologue is unknown, AIT-082 may be able to bind to the enzymatic pocket suppressing or inducing an enzymatic pathway involved in folate degradation or reuse. Folate is a compound required for proper DNA maintenance and found in reduced levels in Alzheimerís disease patients. Though there is evidence suggestive of certain aspects of AIT-082, the definitive molecular mechanism of AIT-082 and PABAís role in it are not fully understood.
These results, though preliminary, point the direction for future research and suggest answers to the processes involved in neurodegenerative disorders. Certainly the binding of properties of PABA differ from AIT-082 and it would be expected to isolate more than one protein using PABA in affinity chromatography. Under the supposition of multiple binding site proteins PABA, of the proteins purified one may be the protein receptor unique for AIT-082 . By using hypoxanthine in affinity chromatography with the proteins isolated in this experiment we could determine which protein binds both ligands. Following the premise of multiple binding site proteins, if either the 38 kDa or 51 kDa bound to a hypoxanthine it would suggest indeed that that particular protein has binding sites for both hypoxanthine and PABA. This would further the case for that specific protein.
A next logical step would be to excise the protein bands and sequence portions of the purified proteins. Crossing these unknown sequences against other mammalian genomes might provide identity for the unknown proteins. Once the protein receptor of AIT-082 has been isolated it could be crystallized, allowing examination of its protein binding sites. By understanding the topography of the receptor, more drugs of higher binding efficiency could be produced. Furthermore, the isolation of a protein so intimately involved in the neuroregenerative process could open biological doors to determining genetic predisposition and to treatments such as gene therapy. It may also reveal ways to treat victims of neurological trauma such as stroke.