Purification and Characterization of Glucose-6-Phosphate Dehydrogenase from Chlamydomonas reinhardtii
Brad Ellison and John H. Williamson
Biology Department, Davidson College, Davidson, NC 28036-1719 USA
ABSTRACT
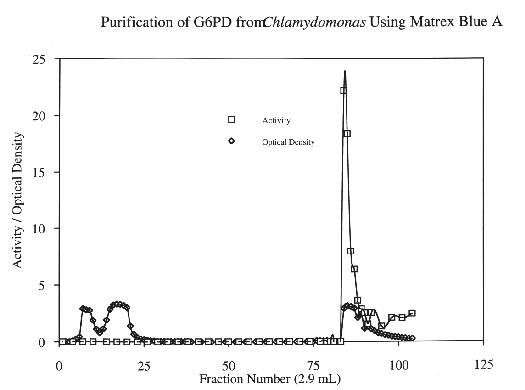
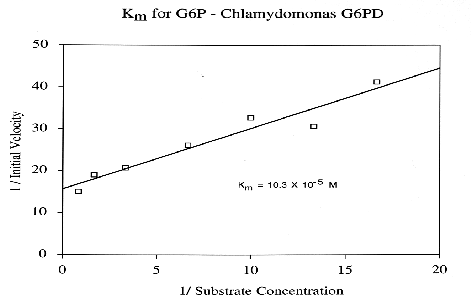
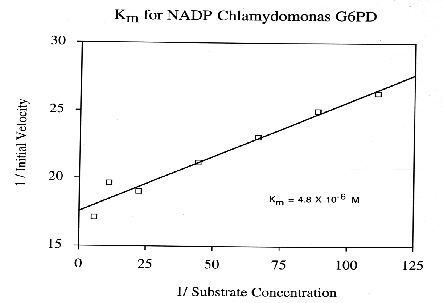
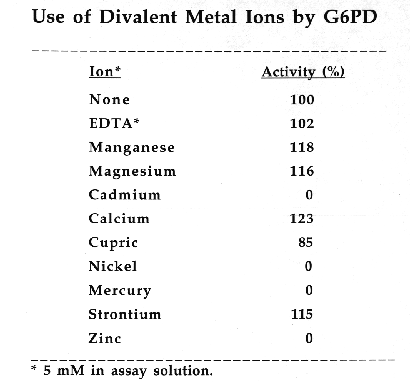
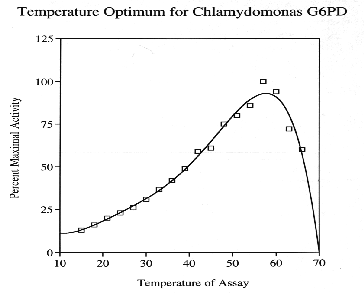
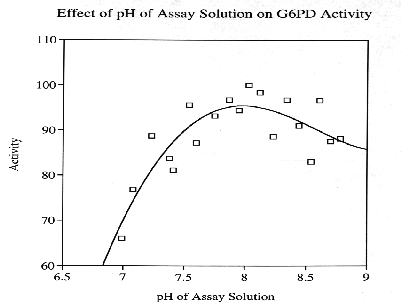
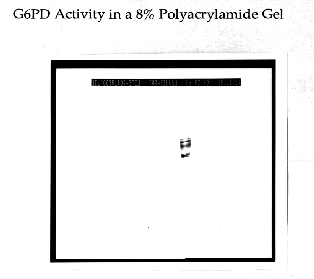
Glucose-6-Phosphate-Dehydrogenase (G6PD) is a NADP-dependent enzyme essential to the oxidative pentose- phosphate cycle. Experimental parameters were designed to isolate and characterize G6PD from Chlamydomonas reinhardtii, an important biological model system. G6PD was purified to apparent homogeneity using Amicon Matrex Gel Blue A. The enzyme appears to be tetrameric with an approximate molecular size of 180 kD. Km values for DL-G6PD and NADP were 103.0 uM and 4.8 uM, respectively. The activity of the enzyme was enhanced in the presence of Mg2+, Mn2+, and Ca2+. However, G6PD cannot utilize Cu2+, Sr2+, or An2+ ions as electron acceptors. Moreover, Cd2+, Hg2+, and Ni2+ were toxic to enzyme activity. G6PD has a broad pH optimum of 7.6 to 8.3 and is stable to a maximum temperature of 60 degrees C.


© Copyright 2000 Department of Biology, Davidson
College, Davidson, NC 28036
Send comments, questions, and suggestions to: macampbell@davidson.edu