Intracellular calcium ions are regulated by calcium pools in the
sarco/endoplasmic reticulum (SER). Calcium pools are composed of three main
groups of proteins: pumps, channels, and buffers. Calcium pumps use ATP to
actively pump calcium ions from the cytosol into the lumen (figure 1). The
calcium pool buffer, calsequestrin (Sacchetto et al., 1995), stores the intracellular
calcium ion supply, while calcium channels release calcium ions back into the
cytoplasm.
Figure 1. The three components necessary for a functional calcium pool. Calcium
pumps actively transport calcium ions from the cytoplasm to the lumen. Calcium
buffers store calcium ions, and calcium channels allow the release of sequestered
calcium ions back into the cytoplasm.
Calcium pools are extremely important in the development of the heart
because calcium ions play a critical role in the excitation-contraction process (Lytton
and MacLennan, 1991). When calcium pools are developed in cardiomyocytes,
intracellular calcium is pumped by SERCA2 from the cytosol into the SER where the
ions are sequestered and later released through calcium channels. Troponin is
located in the sarcoplasm and requires calcium ions to facilitate
activation-contraction of actin and myosin (Lim, et al., 1983). In order for relaxation
to occur, calcium ions must be actively removed from the sarcoplasm.
The sarco/endoplasmic reticulum calcium ATP-ase (SERCA) has three main isoforms. SERCA 1 is expressed in fast-twitch muscle cells (Brandl et al., 1986, 1987). SERCA2 has two different isoforms, SERCA2a and SERCA2b, that differ in the carboxyl terminal region due to alternative mRNA splicing (Campbell, et al 1991). SERCA2a is the calcium pump expressed in cardiac myocytes (Brandl et al., 1987). SERCA2b is known as a general maintenance isoform and is expressed in all cells with the exception of cardiac myocytes and fast-twitch muscle cells (Brandl et al., 1987). SERCA3 is less well documented, but it has been demonstrated to be expressed in non-muscle tissues (Burk et al., 1989; Wu et al. 1988). Related isoforms of calcium pumps are found in the plasma membrane (Kasai 1990). Since the majority of calcium ion regulation of myocytes is associated with the SER, the plasma membrane calcium pumps play a limited role in calcium regulation by actively transporting calcium ions from the cytosol into the extracelluar fluid.
Calcium channels in the SER include the ryanodine receptor and the inositol 1-4-5 triphosphate (IP3) receptor (Fleischer and Inui, 1989). When opened, both receptors allow the release of sequestered calcium pools. During embryonic chicken development, the ryanodine receptor has not been detected until embryonic day four (Dutro, et al. 1993). The ryanodine receptor has been shown to be the primary calcium channel in adult chicken cardiomyocyte SR (Junker, et al. 1994). In contrast, the IP3 receptor is expressed in the intercalated discs and only makes up 1% of the calcium channels in the SER (Kijima, et al. 1993).
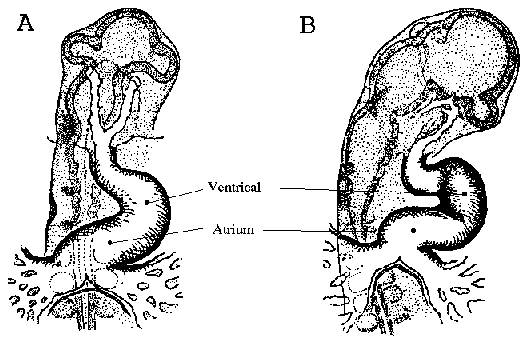
Chicken heart development begins when cardiac primordia fuse, forming a
straight anterior to posterior ventricular tube. As development progresses, the
ventricle begins to fold. Figure 2 is a line drawing adapted from Patten and
Krammer (1949), which depicts the early folding pattern of the ventricle. The first
contractions of the chicken heart occur between Hamburger and Hamilton (1953)
stage 9-10 (embryonic day 1.5/ 29-38 hours) which roughly corresponds to 10-11
somite pairs (Patten and Krammer, 1949).
The purpose of this study was to determine when and in what pattern the
cardiac calcium pump (SERAC2) and the cardiac calcium channels ( the IP3-R and
the Ryanodine-R) are initially expressed in relation to the development of the heart
and the initial cardiac contractions.
Antibody | Dilution | Source |
| Anti-SERCA2 (3H2) (Campbell, et al. 1991) | 5 µg/ml | Dr. Doug Fambrough Johns Hopkins University |
| Anti-Ryanodine
Receptor € (34C) € (110 E) (Dutro, et al. 1993) | 1: 400 1: 100 | Dr. John Sutko University of Nevada Los Vegas |
| Anti-IP3
Receptor Rabbit Serum (Mignery, et al. 1989) | 1:200 | Dr. Pietro DeCamilli Yale University |
| Secondary Antibody Fluorescein-Labeled | 0.05 mg/ ml | Kirkegaard and Perry Labs, MD |
| Bodipy-Ryanodine Incubation Solution |
| Buffered Antibody Solution (PBS +1% BSA+ 0.25% Saponin) |
| Add 1% Triton |
| Bring entire Solution up to 1 mM Bodipy Ryanodine using EtOH or DMSO to initially solubilize the Bodipy Ryanodine. |
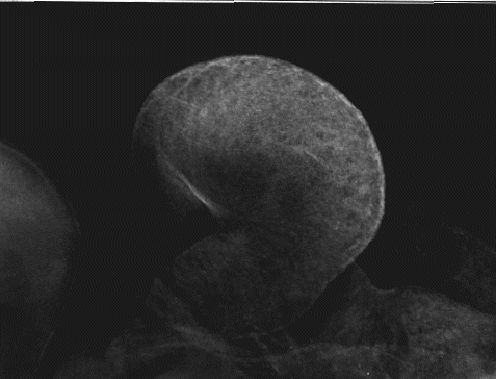
By stage 14, (figure 6; 19 somite pairs; 48-50 hours), labeling of SERCA2 occurs
uniformly throughout the embryonic heart. Notice that the atrium is starting to fold
into position above the ventricle. The labeling on the upper left edge of the photo is
the cranial region. The punctate patterns of SERCA2 are no longer apparent, and
the intensity of labeling seems to be uniform throughout the heart.
Whole embryo immunofluorescent labeling of the ryanodine receptor and
the IP3 receptor were both negative through H/H stage 17 (data not presented).
Since embryos past stage 17 are too think to be used in whole embryo mounting, so
past this stage embryonic hearts were cryosectioned as the search continued.
Cryosectioning Labeling Results
Embryonic cardiac cryosections labeled with anti-IP3 receptor polyclonal
antibodies were negative until stage 21 (H/H) (figure 7), and by stage 23 cardiac
cryosections revealed more intense labeling (data not shown). All attempts to label
embryonic and adult cardiac sections with anti-ryanodine antibodies (34C and 110E)
showed no detectable labeling (data not shown), although 110E and 34C both labeled
adult chicken sartorius tissue very clearly (data not shown). Since antibody labeling
did not work, the newly released bodipy-labeled ryanodine was employed to probe
for the ryanodine receptor. There is no published protocol for this type of labeling.

Bodipy- labeled ryanodine techniques were beginning to show striations on
adult sartorius muscle (data not shown). The technique has not been refined
enough to effectively label embryonic cardiac tissue.
Whole embryo labeling allowed us to observe the intense patch of labeling located in the center of the ventricle (early stage 11). By late stage 11, SERCA2 expression is highest along the lateral wall of the ventricle while expression is not as intense toward central area of the ventricle. This suggest that either the ventricle twists during stage 11, making the central patch appear to have moved the lateral margin of the ventricle or the cells in the central patch area stop expressing SERCA2 by late stage 11, and the cells on the lateral margin start to express SERCA2. The lateral movement of the ventricle seems to be more likely. By stage 13, SERCA2 is expressed evenly throughout the ventricle. This suggests a lateral to medial ventricular expression gradient of SERCA2 from late stage 11 to stage 13 in addition to the anterior to posterior gradient witnessed by Jorgensen and Bashir.
Antibodies 110E and 34C have been successfully used in western blot analysis to detect cardiac ryanodine Dutro et al (1993), but, in this study, no immunofluorescent labeling even in adult cardiac cyrosections was detected. The anti-ryanodine antibodies, 110E and 34C, may not recognize native cardiac ryanodine receptor epitopes. Clearly labeled striations were apparent when 110E and 34C were used to immunoflourescently label adult sartorius skeletal muscle. Since 34C and 110E are functional as demonstrated by the labeled sartorius striations, this suggest that the cardiac ryanodine receptor epitope might be somehow sheltered or contorted such that 110E and 34C can not recognize it unless the ryanodine receptor has by exposed to denaturing agents such as SDS (sodium dodecyl sulfate). Preliminary trails with SDS did not show labeling.
Without calcium channels, calcium pools are incomplete and unable to release intracellular calcium stores, which is one mechanism to facilitate contraction. Dutro et al. (1993), using both anti-ryanodine receptor antibodies (110E and 34C) and ryanodine binding, observed that the ryanodine receptor is present from embryonic day 4 through day 20. They also observed that ryanodine, which can block the ryanodine receptor, causes a slower contraction rate in embryonic hearts. Since the ryanodine receptor is the primary calcium channel in the SER of cardiomyocytes in adults (Junker, 1994), it appears the contraction rate of day 4 embryonic heart and older are dependent on intracellular calcium stores. The IP3 receptor is expressed at stage 21 (day 3.5), but the IP3 receptor is expressed in very limited quantities even in adult cardiac tissue (Kijima, et al.1993). It is doubtful that the IP3 receptor plays a major role in the release of sequestered intracellular calcium stores.
This raises a question: where is the calcium coming from to facilitate contraction between day 1.5, when the contractions begin, and day 4, the earliest the ryanodine receptor is detected? There are two likely answers. First, there could be an undiscovered calcium channel in the SER which is expressed before day 4 that could allow possible intracellular stores to be released. Second, calcium channels on the plasma membrane could allow extracellular calcium to flood in and facilitate contraction. However, one of the most common plasma membrane calcium channels, the nitrendipine receptor, is not expressed until embryonic day 3 (Renaud, et. al., 1984). Also, calcium pumps on the plasma membrane, which pump calcium from the cytosol into the extracellular fluid, could be aiding in early cardiac relaxation. The sodium-calcium exchanger, could also aid in extracting calcium from the sarcoplasma while SERCA2 expression is low in stage 9. However since the ryanodine receptor, which constitutes the vast majority of SER calcium channels, is not detected until embryonic day 4, it seems as though there might be a functional calcium channel on the plasma membrane which allows calcium influxes necessary for contraction.
Recent immunofluorescent studies using anti-calsequestrin antibodies have
show that calsequestrin is initially expressed in cardiac myocytes at stage 14
(embryonic day 2; Permar, personal communication). This suggests that
intracellular calcium storage is capable well before embryonic day 4. Calcium ions
are pumped into the lumen and bind to calsequestrin. If there is no SER calcium
channel, then calcium ion levels in the lumen should rise. Perhaps the
intracellular calcium pools need time to fill their stores with the aid of SERCA2
before the expression of SER calcium channels.
Summary of Calcium Pool Development |
| Stagees: Hamburger and Hamilton | Stage 10 (Day 1.5) (33-38h) | Stage 11 (Day 1.75) (40-45h) | Stage 14 (Day 2.25) (50-52h) | Stage 17 (Day 2.5) (52-64h) | Stage 21 (Day 3.5) (84h) | Stage 24 (Day 4) (96h) |
| Protein Expression | Calcium Pump Begins | Calcium Pump Spreads | Calcium Pump Spreads Throughout Entire Heart | Calcium Pump Continues | Calcium Pump Continues | Calcium Pump Continues |
| - | - | - | Calsequestr
in Begins | Calsequestrin Throughout Heart | Calsequestrin Continues | Calsequestrin Continues |
| - | - | - | - | - | IP3
Receptor Begins | IP3
Receptor Present |
| - | ? | ? | ? | ? | ? | Ryanodine
Receptor Present (Dutro et al., 1993) |
Future analysis will attempt to achieve immunofluorescent labeling of the
ryanodine receptor before embryonic day 4 (stage 24). Since immunofluorescence
shows more data than western blot analysis, it is possible that the Ryanodine
receptor is present early than stage 24. Efforts will center around using brief SDS
washes to expose the epitope of the cardiac ryanodine receptor (Brown et al, 1996), so
that hopefully 110E and 34C will show labeling. Also, future research will seek to
optimize bodipy-ryanodine labeling methods, thus providing researchers with a
potentially useful tool for observing the ryanodine receptor.
Learning more about calcium pool components and interactions could greatly
enhance our understanding of cardiac development, SER development, and ion
regulation. Calcium regulates such a broad variety of critical life functions and
interacts with so many different proteins that is of considerable importance to
understand role of calcium ions in this complex and wonderful experience we call
life.
Brandl, C. J., Green, N. M., Korczak, B., MacLennon, D. H. (1986) Two Ca+2 ATPase genes: homologies and mechistic implications of deduced AA sequences. Cell. 44:597-607.
Brandl, C. J., DeLeon, J., Martin, D. R., and MacLennan, D. H. (1987) Adult forms of the Ca+2 ATPase of the SR, expression in developing skeletal muscle. J. Biol. Chem. 266, 3768-3774.
Burk, S. E., Lytton, J., Maclennan, D. H., and Shull, G. E. (1989) cDNA cloning, functional expression, and mRNA tissue distribution of a Third Organellar Ca+2 pump. J. Biol Chem. 264:18561-18568.
Campbell, A. M., Kessler, P. D., Sagara, Y., Inesi, G., and Fambrough, D. M. (1991) Nucleotide sequences of avian cardaic and brain SR/ER Ca+2 ATPases and functional comparisons of fast twitch Ca+2 ATPases. J. Biol Chem 266: 16050-16055.
Dutro, S. M., Airey, J.A., Beck, C. A., Suktko, J. L., and Trumble, W. R., (1993) Ryanodine receptor expression in embryonic avian cardiac muscle. Developmental Biology 155: 431-441.
Fleisher, S., and Inui, M. (1989) The biochemistry and biophysics of excitation-contraction. Annu. Rev. Biophys. Biophys. Chem. 18: 333-364.
Gilchrist, J., Czubryt, M., Pierce, G. (1994) Calcium and calcium binding proteins in the nucleus. Molecular and Cellular Biochemistry 135:79-88
Hamburger, V., and Hamilton, V. (1951) A series of normal stages in the development of the chick embryo. J. Morphol. 88:49-92.
Jorgensen, A.O. and Bashir R. (1984) Temporal appearance and distribution of Ca+2 +Mg+2 ATPase of the sarcoplamic reticulum in developing chick myocardium as deterimined by immunoflourescence labeling. Development Biology 106: 156-165.
Junker, J., Sammers, J., Madhabanda, S., Meissner, G. (1994) Extended Junctional Sarcoplasmic reticulum of avian cardiac muscle contains functional ryanodine receptors. J. Biol Chem. 269 (3): 1627-1634.
Kamino, K. (1991) Optical approaches to ontogeny of electrical activity and relted functional organization during early heart development. Physiological Reveiws 71: 53-88.
Kasai M., Muto, S. (1990) Ca +2 pumps and Ca+2/H+ antiporter in plasma membrane vesicles isolated by aqueous phase partitioning from corn leaves. J. Mem. Biol. 114:133-142.
Kijima, Y., Saito, A., Jetton, T., Magnuson, M., and Fleischer, S. (1993) Different
intracelluar localization of inositol 1,4,5-triphosphate and ryanodine receptors in
cardiomyocytes. J. Biol Chem. 268 (5): 3499-3506.
Lim, S., Woodroofe, M.W., and Lemonski, A. (1983) An analysis of contractile
proteins in developing chick heart by SDS polyacrylamide gel electrophoresis and
electron microscopy. J. Embroyol. Exp. Morphol. 77:1-14.
Lytton, J., and MacLennan, D. H. (1988) Molecular clones of cDNAs from human kidney coding for two alternatively spliced products of the cardaic Ca 2+ATPase gene. J. Biol Chem. 263:15024-15031.
Lytton, J., and Nigam, S.K. (1992) Intracelluar calcium: molecules and pools. Curr. Opin. Cell Biol. 4:220-226
Migery, G. A., Sudhof, T. C., Takei, K., and DeCamilli, P. (1989) Putative receptor for
inositol 1,4,5-Trisphosphate--Similar to ryanodine receptor. Nature 342: 192-195.
Patten, B. M., and Kramer, T. C. (1949) Initiation and early changes in the character
of the heart beat of vertebrate embryos. Physiol. Rev. 29: 31-47.
Putney, J. W. Capacitive calcium entry revisited. Cell Calcium 11:611-624, 1990.
Renaud, J., Kazazoglou, T., Schid, A., Romey, G., Ladzdunski, M. (1984) Differentiation of receptor sites for nitrendipine in chick hearts and physiological relation to the slow Ca +2 channel and to exctation-contraction coupling. E. J. Biochem. 139: 673-681.
Sacchetto, R., Cliffer, K. D., Podini, P., Villa, A. T., Christensen, B. N., Volpe, P. (1995) Intracellular Ca +2 stores in chick cerebellum perkinje neurons: ontogenetic and functional studies. A CELL August 29, session 5.
Verboomen, H., Wuytack, F., Van Den Bosch, L., Mertens, L., and Casteels, R. (1994) The functional importance of the extreme C-terminal tail in the gene 2 organellar Ca +2- transport ATPase (SERCA2a/b). Biochem. J. 303: 979-984.
Wu, J., London, J., Zecevic, D., Hopp, H., Cohen, L., and Chun, X. (1988) Optical
monitoring of activity of many neurons in invertebrate ganglia during behavior.
Experientia Basel 44:369-376.
Zimmerman, D. (1989) Intracellular calcium: ubiquitous, vital, deadly. Mosaic 20
(3).
© Copyright 2000 Department of Biology, Davidson
College, Davidson, NC 28036
Send comments, questions, and suggestions to:
macampbell@davidson.edu