Protocols for GCAT
Growing Yeast
Media Recipes:
Use one of the following media for growing wild type yeast strains. Note that pre-made media can be purchased from many companies, such as Bio101.
- To make 1 liter of YD media (also called YEPD and YPD): (from Doug Boreham <borehamd@aecl.ca> and Karen Bernd <kabernd@davidson.edu>)
- 1% Yeast Extract (Bacto) = 10 g per liter
- 2% Bactopeptone = 20 g per liter
- 2% Dextrose (glucose) = 20 g per liter
- Place ~500ml of H2O and a stir bar into the flask
- Stir on stir plate until all goes into solution (note if making plates agar will NOT go into solution)
- Transfer to graduated cylinder. Add H2O to 1L. Pour back into 2L flask.
- Cover flask with aluminum foil. Add small piece of autoclave tape. Autoclave at 121° C for 25 min, liquid cycle (slow exhaust).
- Media will caramelize (turn brown due to "cooked" sugar) if left in autoclave too long. Slight caramelization will not hurt the media.
- Allow media to cool then store in refrigerator or use when room temp.
- To make 1 liter of YES media: (from Doug Boreham <borehamd@aecl.ca>)
- Yeast Nitrogen Base with Amino Acids = 6.7 g/l
- Dextrose (glucose) = 2.0 g/l
- Sodium Succinate = 10.8 g/l
- Yeast Extract = 1.0 g/l
- (Note: To make liquid growth media into solid media for petri dish plating add 2% agar (20 g/l)).
Return to Top of Page
Media Preparation
- Liquid
- Solid
- Liquid Media Preparation:(from Doug Boreham <borehamd@aecl.ca>)
- We usually make 10 liters of media at a time by adding the appropriate amounts of the above reagents to 10 liters of distilled water.
- Using a magnetic stirrer, dissolve the reagents (stir for at least 10 minutes).
- Pour about 400 ml of media into 500 ml glass bottles.
- Place the caps on loosely and autoclave the bottles for 40 minutes. Use slow exhaust on the autoclave or media can boil over at the end of the cycle.
- The media is cooled in a sterile hood until warm (about 50° C) and then the caps are sealed tightly.
- The media is stored at 4° C in a refrigerator until use.
- Solid Media Preparation:(from Doug Boreham <borehamd@aecl.ca>)
- Follow the same technique to make solid agar plates (media contains 20 g/l agar).
- Once the media has cooled to about 70° C, it can be poured directly from the bottle into sterile plastic petri dishes in a sterile hood (you can do it on the bench if the lid is held over the dish when pouring and provided the air currents are not strong).
- Fill petri dishes to about 1/3 capacity (just enough to cover the bottom of the dish by swirling the petri dish to spread the hot agar).
- The plates can be stacked and cooled to room temperature in a sterile hood. Usually, after the plates are poured the sterile hood can be turned off to avoid air currents drying out one side of the plate (it shrinks and is thinner on one side making plating more difficult).
- Once the plates have solidified and are at room temperature, they are place back into their plastic bags and the bag is taped closed.
- The plates can be stored at room temperature for months.
Return to Top of Page
Strains:
- The strain of yeast that was sequenced was S288C. If you want your students to use the same genome for theoretical reasons or if your students might do some sequencing and you want it to be the same as in the database, then use the same strain. Other strains will contain different genotypes, which may or may not be desirable for your experiements.
Return to Top of Page
Growth Conditions
Starting a yeast liquid culture from a frozen stock:(from Karen Bernd <kabernd@davidson.edu>)
The following are protocols provided to undergraduate researchers using yeast for the first time. They provide every detail I could think of. (K.B.)
Notes:
- All materials used must be sterile (Including inoculating "toothpicks")
- YEPD is yeast "rich media". The media used will vary if yeast strains need to remain under selection.
- Aeration is important so cultures should be placed in tubes or flasks that provide extra space and should be agitated during incubation ("roller barrel" is best but racks or tubes in a shaking incubator [175rpm or better depending on tube/flask used])
- Optimal growing temperature for wild type yeast is 30° C. They can be grown at room temp if necessary but they will not be happy at
37° C)
- Aliquot 3 ml of YEPD into a sterile test tube (10 cm x 1 cm).
- Remove frozen culture from -80° C and place on ice. Remove cap from culture and scrape up a small amount of the frozen culture using sterile toothpick.
Notes:
- wooden applicator stick (long toothpick) or sterile pipet tip can also be used
- frozen cultures can be reused-replace the cap and place back in the -80°C for 3-5 years)
- Transfer the scraping to the test tube/media. Make sure that tip of toothpick touches media. Very little culture needs to be transferred for successful inoculation.
- Place cap on test tube and shake at 30° C for ~48hrs.
- This stock culture can be stored at 4° C for 2 weeks and used to inoculate other cultures.
- Starting a yeast stock plate from frozen stock or liquid culture:(from Karen Bernd <kabernd@davidson.edu>)
- On the "agar side" of a YEPD plate, write the name of the culture, date it was streaked, and your initials.
- A) From Frozen culture:
- Remove frozen culture from -80° C and place on ice.
- Remove cap from culture and scrape up a small amount of the frozen culture using sterile toothpick (see notes in #2 above)
- Recap frozen stock and quickly replace in freezer!
- B) From Liquid stock:
- Swirl stock culture to mix yeast (settled at bottom) with media.
- Using pipetman and sterile tip remove 3 µl of culture.
- Touch toothpick or pipet tip to YEPD plate. Do not gouge agar. Make ~2 cm line on plate.
- Turn plate 90°. Using new sterile toothpick draw zig zag line that passes through first line one time.
- Repeat step above 2 more times, making new line each time with a new sterile toothpick passing through last zig zag one time. (See drawing below)
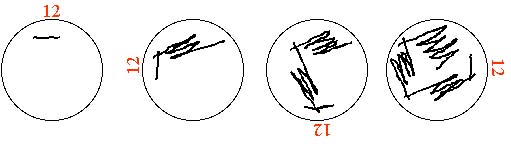
(Trying to separate single colonies: changing toothpicks and turning the plate means that you pick up and spread out fewer cells with each pass)
- Replace lid on YEPD plate. Place in 30° C incubator with agar side up for ~2-3 days.
- Plate can be stored at 4° C (refrigerator) for 1 month
Return to Top of Page
Biology Home Page


© Copyright 2001 Department of Biology, Davidson College, Davidson, NC 28036
Send comments, questions, and suggestions to: macampbell@davidson.edu
