Figure 1:
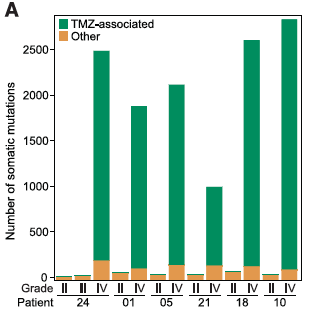
- Panel A: Represents the number of somatic mutations exclusive to the original tumor, exclusive to the recurrence, and found in both. The bars are labeled with patient number and sorted by the histological grade (how similar the tumor tissue is to healthy tissue—the higher the grade, the worse the tumor) of the recurrent tumors; TMZ treated patients are marked with a line. Five of the eight TMZ treated patients shown have over 1000 somatic mutations in their recurrent tumors, with several nearing 3000. In patients not treated with TMZ, the numbers of somatic mutations exclusive to the recurrent tumor are much lower, generally staying below 100. In every case, the number of mutations exclusive to the recurrence is greater than the number exclusive to the original tumor, while the number shared tends to fall between the two on average (for the TMZ-treated patients with >1000 mutations in the recurrence, there are much fewer mutations shared between the original and recurrent tumor); however, this is not marked by statistical significance.

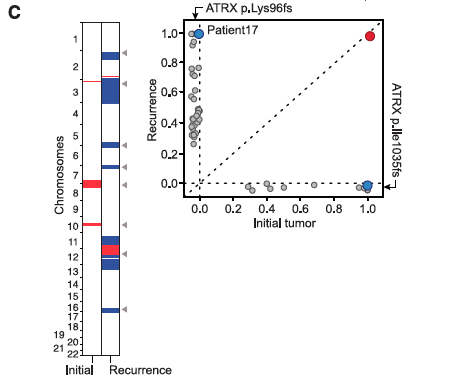
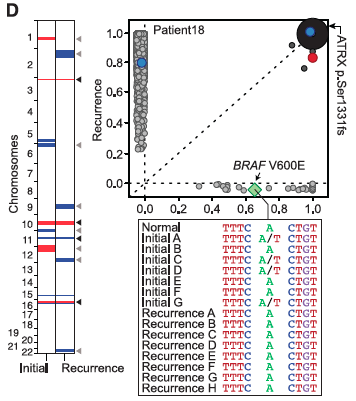
- Panel B-D: Contain diagrams explaining on which chromosomes mutation occurred, differentiating between initial recurrent tumors, marking gained/lost mutations in red and blue, and denoting exclusive mutations with light grey arrows, shared with dark grey. Each panel also includes a graph similar to a dot-plot, showing whether a mutation is shared or private and at what frequency it occurs in each tumor. If a mutation is present in all the cells of both tumors, it is noted with a point with a larger radius.
- Panel B shows Patient 27, who has more mutations private to the recurrence that are present in a higher fraction of cells than in the initial tumor; Patient 27 also has a mutation in IDH1 shared by all cells, and a few other shared that occur in all cells of the recurrence but only between 70-90% of the initial tumor.
- Panel C is Patient 17, who has only one shared mutation (IDH1) between the initial and recurrent tumors; again, far more mutations exist in the recurrent than in the initial tumor, and these vary in frequency within the tumors themselves.
- Panel D relates the mutations of Patient 18, who has a huge number of mutations in the recurrent tumor relative to the initial tumor. Again, IDH1 is shared by both, and Patient 18 has a few other shared mutations that are in high frequency for both recurrent and initial tumors. Panel D also includes the sequence for the BRAF V600E (a gene related to signaling for cell growth) with mutations that were sampled from geographically different locations in initial and recurrent tumors, as well as the normal sequence.
Figure 2: shows us MRI scans of two different patients at various stages of recurrence, denoting how much time passed between recurrences, and when TMZ chemotherapy occurred. It also includes a phylogenetic tree of tumors, denoting the mutations that separate the initial tumor and the recurrences.
- Panel A: Shows us Patient 17, who had 12 months of TMZ therapy and a total of 30 months between the initial tumor and the recurrence. Patient 17 had a single mutation to IDH1 common to both the initial tumors, and the 4 recurrences. Recurrences developed their own mutations, splitting into the lineage that led to Recurrences C and A, and a separate lineage that became Recurrence B and later D.
- Panel B: Shows us Patient 04, who went through 15 months from initial to Recurrence 1, 20 to Recurrence 2, and 9 to Recurrence 3. Two rounds of TMZ chemotherapy (7 and 6 months respectively) occurred between Recurrences 1 and 2, and 2 and 3. In this case, the initial tumor again has its own lineage; Recurrence 1 is more closely related to the initial tumor than either of the other recurrences, which evolved Recurrence 2 and later 3. As with every other patient, a mutation to IDH1 is ancestral to all tumors.
Figure 3:
- Panel A: Shows us the numbers of TMZ-associated and other mutations in recurrent tumors of six TMZ-treated patients, each of which had at least one Grade IV recurrence; for all samples shown, the Grade IV recurrence had huge numbers of somatic TMZ-associated mutations (most ranging from 1500-2500), as compared to under 250 “other” mutations across the board. Grade II recurrences had no TMZ-associated mutations and relatively few “other” mutations compared to Grade IV recurrences.
- Panel B: Depicts the high association of hypermutation in the AKT-mTOR pathway and the RB pathway in TMZ treated patients who had Grade IV recurrences. Of the hypermutations that occurred in the TMZ treated patients, the majority appear to have been TMZ-associated.
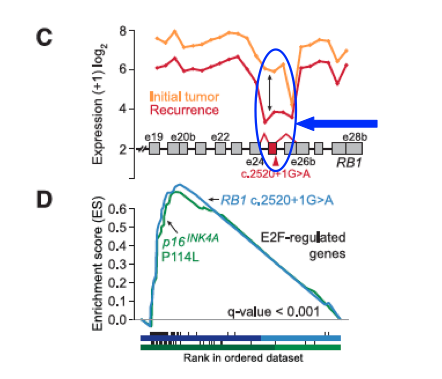
- Panel C: Notes the dramatic change in the RB pathway associated with the recurrent tumor in Patient 01; the recurrence has a mutation that alters the splicing of the mRNA, causing a large drop in expression (see circled region).
- Panel D: Shows us that mutations in the RB pathway, associated with TMZ, cause an increase in the activity of genes usually down-regulated by RB1 and up-regulated by E2F, for both patients 1 and 10. RB1 and E2F are both part of the RB tumor-suppressor pathway.
- Panel E: Allows us to view physical samples of the first recurrent tumor in Patient 01, stained for easier viewing. The two regions of the tumor differ in that one has a TMZ-associated mutation MTOR S2215F, while the other is wild-type for MTOR (a gene in the AKT-mTOR pathway). There is a clear difference between two regions of the tumor, as the MTOR S2215F region proliferated more than the wild-type region. Recall that the AKT-mTOR pathway normally regulates apoptosis, and hyperactivation of the pathway reduces apoptosis in cancer cells.