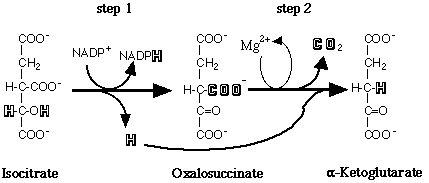
Figure 1. IDH catalyzes the sequential dehydrogenation and decarboxylation of isocitrate to a- ketoglutarate.
Assigned Reading: "Enzymes..." pp 128 - 131
Stop @ bottom of pg. 131.
144, Fig 7.3 [reduction of NAD+]
Bring a calculator to lab
Goals of this Lab
With this laboratory, we begin a three week study and discussion of some of the properties of NADP+-dependent IDH. The goals of these laboratory sessions are:
1. Learn spectrophotometric analyses of enzyme activity.
2. Determine how the amount of enzyme in the assay affects the
rate of
activity.
3. Determine how the amount of substrate in the assay mixture
affects rates
of activity of an enzyme.
4. Determine the effects of environmental conditions on enzyme
activity.
5. Learn how to organize our data into tabular and graphic form.
I. Introduction
Enzymes are biological catalysts with remarkable power, increasing reaction rates by at least a million-fold. They do this by lowering activation energies, allowing chemical reactions to proceed under physiological conditions. Enzymes are highly specific as to substrates and reactions catalyzed. They are usually proteins, although some are other types of biological molecules. Enzymes function best in dilute aqueous solutions under limited conditions of temperature, pH and salt concentration. Some enzymes require one or more non-protein components called "coenzymes" and "cofactors"; a coenzyme is an organic molecule, while a cofactor may be a metal ion. Some enzymes simultaneously require both a cofactor and a coenzyme. Isocitrate dehydrogenase [IDH] is one of these, requiring both NADP+ as a coenzyme and a divalent metal cofactor, Mg2+ or Mn2+.
IDH is a ubiquitous enzyme, being found in all living organisms and has two catalytic activities [Figure 1]. As its name implies, IDH removes hydrogens from its substrate, isocitrate; in addition, it is a decarboxylase, removing a CO2 from the six-carbon substrate to generate a five-carbon product, a-ketoglutarate .

Two distinct forms of IDH are found in higher organisms. They differ in their distribution within the cell and in coenzyme requirements. The soluble form of IDH requires NADP+ as its coenzyme [Figure 2]. The NADP+-dependent form of IDH is considered to be the only IDH in bacteria and cyanobacteria and is the most prevalent form of IDH in most plants and animals. In higher organisms, this form appears to be found in all organs and tissues. This form of IDH is used in lipid synthesis. The NAD+-dependent form of IDH is limited to eukaryotic organisms and is localized in mitochondria. You may already know this form of IDH from previous study of the Krebs cycle. Both forms of IDH require a divalent metal ion.
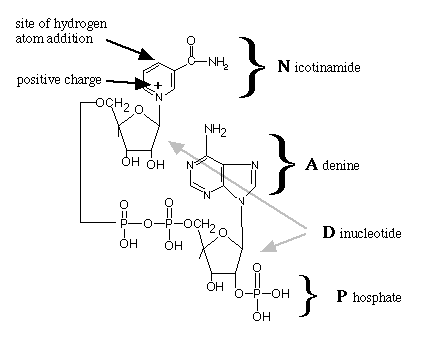
Figure 2. The molecular structure of NADP+.
The reactive site is where the hydrogen atom will be added to
convert NAD+ to NADPH. In NAD+ , the phosphate group is replaced with an H.
This diagram illustrates what the letters N-A-D-P represent.
NEWS ITEMS: In June, 1996, a team of researchers found a species of voles that is resistant to mutations caused by radiation. When they analyzed their cells, they found that the voles had elevated levels of IDH, which they believe is protecting them from radiation-induced mutations. (See summary in Science. Vol 273. 19 July, 1996)
NADP+-dependent IDH activity is especially high in cardiac tissue and is one of the enzymes monitored in the blood of heart attack patients. Detectable IDH activity in the arterial blood suggests severe tissue damage with leakage of the soluble (cytosolic) IDH into the blood system.
IDH activity is routinely measured using
a spectrophotometer to monitor the reduction of NADP+ to NADPH. While running assays, the spectrophotometer
is set at 340 nm, the absorption maximum of NADPH (and results
from last week's lab). Assays are performed at a standard temperature,
usually 25° C to 30° C.
Before a scientist begins an experiment, he or she must first
define a problem and suggest possible explanations based upon
previous knowledge or observations. In other words, develop an
hypothesis, which might be considered an "educated guess"
or a tentative explanation as to the cause and effects relating
to that problem. A good hypothesis is one that is testable and
fosters predictions that consider one variable at a time. The
hypothesis may turn out to be incorrect, but it is a good hypothesis
if it can be tested. In fact, an hypothesis that can not be tested
is useless to science - it may be good philosophy, but not good
science. Hypotheses can not be proven to be correct - they may
be tested extensive and rigorously and they may be proven to be
incorrect, but they can never be proven to be true.
Our scientist must first define a problem and then develop an
hypothesis. Next one must devise predictions that will hold, or
will not hold, if the hypothesis were true. These predictions
lead to experiments. Many experiments may be possible, and may
all be tried eventually; however, it is important to perform one
discrete experiment at a time. After designing an experiment,
our scientist must outline a series of logical procedures to be
completed in the laboratory or in the field. This written sequence
of steps is called a protocol. A well planned protocol
will include the following elements:
1. An outline of the sequence of detailed procedures.
2. Calculations of volumes, concentrations, etc., of all reagents to be used.
3. Tables constructed for recording data.
4. Procedures for testing and organizing data for presentation.
Hypothesis 1: A successful assay for IDH activity simultaneously requires enzyme, isocitrate and NADP+.
Hypothesis 2: Under ideal conditions, IDH activity will be linear for at least three minutes.
To test your hypotheses, you will need to set up assays as in Table 1. You should ask yourself "What is the purpose of each assay?" You should also ask why Assays 5 - 7 are identical.
| Well | Buffer | NADP+ | Enzyme | Isocitrate |
| A1 | 200 | 0 | 0 | 0 |
| A2 | 180 | 10 | 0 | 10 |
| A3 | 180 | 10 | 10 | 10 |
| A4 | 180 | 0 | 10 | 10 |
| A5 | 170 | 10 | 10 | 10 |
| A6 | 170 | 10 | 10 | 10 |
| A7 | 170 | 10 | 10 | 10 |
All volumes are in µl. In this experiment, you will initiate the reactions by adding 10 µl of enzyme solution as the last step. You will use a multi-tip pipet, at the plate reader, to add enzyme to all wells.
1. Use the P-200 micropipet to add Assay Buffer to the indicated
wells.
2. Use the P-20 micropipet to add 10 µl of NADP+ to all
wells, except A1 and A4.
3. Use the P-20 micropipet to add 10 µl of isocitrate to
all wells, except A1 and A3.
4. Place the microplate in chamber of the plate reader.
5. Use the Multi-8 micropipet to add 10 µl of IDH to all
wells, except A1 and A2.
6. Activate the plate reader.
7. After printing, remove your plate from the plate reader.
8. Retrieve your data from the printer.
9. Return to your station and organize your data in the Table
1a (below).
10. Prepare a graph of your data.
| Time, min | Well A2 | Well A3 | Well A4 | Well A5 | Well A6 | Well A7 |
| 0 | ||||||
| 0.5 | ||||||
| 1 | ||||||
| 1.5 | ||||||
| 2 | ||||||
| 2.5 | ||||||
| 3 |
Considerations - Experiment 1
Compare your data from Wells A 1 through A 7. Was there activity in Wells A 1 - A 4? Was there activity in Wells A 5 - A 7? Was activity the same in Wells A 5 - A 7? Was activity linear for three minutes? If not, explain your observations. Do your data support your hypotheses? If not, how will you change the protocol? Determine the "corrected" reading for each assay by subtracting the reading from the "control", Well A 1. (Would Well A 2, A 3 or A 4 provide better "control" data?)
Construct a graph that visually portrays your data from Wells A 2 - A 7 by plotting absorbance as a function of time (in minutes). The initial rate of a reaction may be determined from the slope of the line joining each successive point. This will be graph 1.
Use the directions for Cricket Graph in Appendix A in the
back of the lab manual.
Problem: What is the relationship between the rate of a reaction and the amount of enzyme in the assay solution when substrate and coenzyme are abundant (non-limiting)? This question might become "In subsequent experiments, how much enzyme solution should I use in each assay?"
Hypothesis: IDH activity will vary directly with the amount of enzyme in each assay.
To test this hypothesis, you will need to follow a protocol that holds all conditions constant except the amount of enzyme added to each assay. All tests should be run more than once; routinely, enzyme assays are run "in triplicate". For example, Wells B 1, B 2 and B 3 in Table 2 are triplicate assays containing 5 µl of IDH. Set up reactions as per Table 2.
|
Wells* B1-3 |
Buffer 175 |
NADP+ 10 |
Enzyme 5 |
Isocitrate 10 |
| B4-6 | 170 | 10 | 10 | 10 |
| C1-3 | 165 | 10 | 15 | 10 |
| C4-6 | 160 | 10 | 20 | 10 |
| D1-3 | 200 | 0 | 0 | 0 |
Procedure
1. Use the P-200 micropipet to add Assay Buffer to the indicated
wells.
2. Use the Multi-8 micropipet to add 10 µl of NADP+ to all
wells.
3. Use the Multi-8 micropipet to add 10 µl of isocitrate
to each well.
4. Place the microplate in chamber of the plate reader.
5. Use the three tips on the Multi-8 micropipet to add the appropriate
volume of IDH to the wells.
6. Activate the plate reader.
7. After printing, remove your plate from the plate reader.
8. Retrieve your data from the printer.
9. Return to your station and organize your data in Table 2a
(below).
10. Prepare a graph of your data.
| Time, min | 5 ul of IDH | 10 ul of IDH | 15 ul of IDH | 20 ul of IDH |
| 0 | ||||
| 0.5 | ||||
| 1 | ||||
| 1.5 | ||||
| 2 | ||||
| 2.5 | ||||
| 3 |
Considerations - Experiment 2
Compare the data from Wells B 1 through C 3. Was there activity in all wells? Did activity vary with the amount of enzyme in each assay? Was activity the same in the three wells with the same amount of enzyme? Was activity linear for the first three minutes for each volume of enzyme? If not, explain your observations. Do your data support your hypothesis?
Determine the mean activity for each set of triplicate assays. Construct a graph to portray your data. Compare activity with the volume of enzyme in the assay solution. [Hint - take advantage of Cricket Graph's ability to genterate a formula for the best fit line: y = mx + b; b is the Y intercept and m is the slope or change in absorbance over time which is the definition of activity.] This will be graph 2A.
Construct another graph that compares volume of enzyme the slope of the three lines (slope equals enzyme activity) from your previous graph. You may use the table below to collect and organize the data. This new graph will be graph 2B. What conclusions can you reach from your results?
|
|
|
|
Problem: What is the relationship between the rate
of a reaction and the amount of isocitrate in the assay solution
when the amounts of IDH and NADP+ in the assay are held constant?
Before you start this experiment, develop an hypothesis and sketch
a graph predicting the relationship of activity vs isocitrate
concentration.
Procedure: To test your hypothesis, you will need to follow a protocol that holds all conditions constant except the amount of isocitrate added to each assay. Table 3 outlines such a protocol using five concentrations of isocitrate. Each concentration is tested in triplicate. Add reagents to your wells as listed from left to right.
|
|
||||
|
|
|
|
|
|
|
|
|
1* 10 |
|
|
|
|
|
2* 10 |
|
|
|
|
|
3* 10 |
|
|
|
|
|
4* 10 |
|
|
|
|
|
0 |
|
|
*The concentration of these isocitrate solutions will be provided by the instructor. The second number refers to the volume [ul] to be used.
1. Use the P-200 micropipet to add Assay Buffer to the indicated
wells.
2. Use the P-20 micropipet to add 10 µl of the different
concentrations of isocitrate to the wells,
as indicated.
3. Use the Multi-8 micropipet to add 10 µl of NADP+ to all
wells.
4. Place the microplate in chamber of the plate reader.
5. Use the Multi-8 micropipet to add 10 µl of IDH to all
wells.
6. Activate the plate reader.
7. After printing, remove your plate from the plate reader.
8. Retrieve your data from the printer.
9. Return to your station and organize your data in Table 3a
(below).
10. Prepare a graph of your data.
|
|
mM |
mM |
mM |
mM |
mM |
| 0 | |||||
| 0.5 | |||||
| 1 | |||||
| 1.5 | |||||
| 2 | |||||
| 2.5 | |||||
| 3 |
Considerations - Experiment 3
Compare the data from your experiment. Determine the activity for each concentration of isocitrate by constructing a graph and generating the best fit lines and equations this will be graph 3A. Next, construct a second graph that compares activity as a function of isocitrate concentration. This will be graph 3B. Do your data support your hypothesis? Is the relationship between activity and concentration of substrate linear? Explain this relationship, referring to graphs 3A and 3B.
In next week's lab, you will study the effects of environmental
conditions on enzyme activity. Each team of students will design
an experimental protocol to study one of the following:
1. What are the effects of 37°C on the stability of
IDH?
2. What are the effects of pH of the assay solution?
3. What are the effects of NADP+ concentration?
4. What are the effects of different divalent metal ions?
5. What are the effects of varying salt concentrations?
6. Which species or tissues have the most activity?
Before leaving lab today, each group will complete the following:
1. Develop a clear, concise and simple hypothesis about the
effects of one of the above
environmental conditions upon enzyme activity.
2. Design an experiment to test that hypothesis.
3. Prepare a protocol to carry out that experiment.
© Copyright 2000 Department of Biology,
Davidson College, Davidson, NC 28036
Send comments, questions, and suggestions to: macampbell@davidson.edu