Davidson Molecular Biology Page
Back to Andy Kazama's Molecular Homepage
This Website is for a class project and NOT a commercial site
Cytochrome C Oxidase Polypeptide Subunit II
| Cytochrome C Oxidase is a vital part of almost every living creature. This metabolic protein catalyses the gerneration of a proton electrochemical gradient across the cell membrane (Ostermeier et al, 1996). It is divided into three subunits (I, II, II) in mammalian systems, but can be composed of up to 13 subunits in other systems (Ostermeier et al, 1996). Cytochrome C Oxidase Subunit II (COX) contains the binuclear CuA center, which transfers electrons from COX and transfers them to the oxygen-binding site. The Haem/copper terminal oxidases catalyse the reduction of dioxygen to water. Click here to see a rasmol image of mammalian Cytochrome C Oxydase Polypeptide II, and then compare it with the entire catalyst by Clicking Here. |
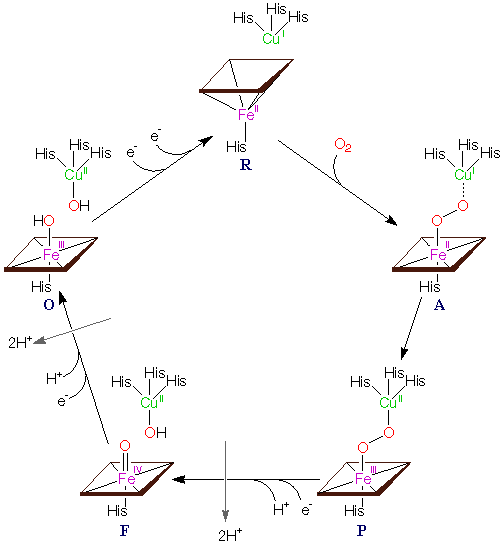 |
| Figure 1. This shows the catalytic cycle for Cytochrome
C Oxidase
Figure complements of http://www-bioc.rice.edu/~graham/CcO.html |
| To compare different forms of this protein you can click on the different hyperlinks available below all of them contain amino acid sequences and cDNA sequences. |
| Yeast (Saccharomyces cerevisiae)
Horse (Equus caballus) Frog (Xenopus laevis) Tarsius (Tarsius syrichta) Cat (Felis catus) |
References
1. Ostermeier, C., Iwata, S. and Michel,
H.(1996) Cytochrome c oxidase. Curr. Opin. Struct. Biol. 6, 460-466.
2. Hill, B.C. (1994) Modeling the
sequence of electron transfer reactions in the single turnover of reduced,
mammalian cytochrome c oxidase with oxygen. J. Biol. Chem. 269, 2419-2425.
© Copyright 2000 Department of Biology, Davidson College, Davidson,
NC 28036
Send comments, questions, and suggestions to:ankazama@davidson.edu