A reporter protein is one that you can detect easily and is not present normally in your research system. For example, you would not want to use a bacterial protein as your reporter if you were studying bacteria. There are many reporter proteins in use: β-galactosidase (encoded by the bacterial gene lacZ), luciferase (from lightening bugs), chloramphenyl acetyltransferase (CAT; from bacteria) used extensively in the single gene genomic circuit chapter, GUS (β-glucuronidase) commonly used in plants) and green flourescent protein (GFP; from jelly fish).
If you wanted to know whether a particular promoter were activated or not, you'd like to be able to determine its activity without having to measure mRNA levels which is very difficult. You'd like to have an easy way to see that a promoter is activated. Or perhaps you would like to be able to watch your favorite protein as it traveled around a cell performing its cellular role. One protein has been very successful at both of these molecular methods - Green Flourescent Protein (GFP).
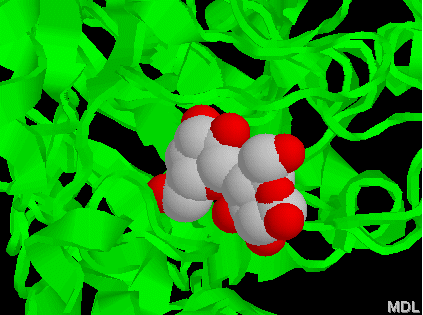
One of the first reporter proetins, and still very popular, is β-galactosidase which is encoded by the bacterial gene lacZ. In bacteria, β-galactosidase cleaves the disaccharide lactose (sugar found in milk) into glucose and galactose (figure 1). β-galactosidase cleaves the colorless substrate X-gal (5-bromo-4-chloro-3-indolyl-β -galactopyranoside; figure 2) into galactose and a blue insoluble product of the cleavage. Because most genomes do not contain lacZ, it can be used as a reporter gene (e.g. blue/white selection).
Figure 1. Lactose bound to one chain of
E. coli β-galactosidase.
If you want to see a chime version, you may click
here. The file is about 3MB so it may take a while to download to your computer.
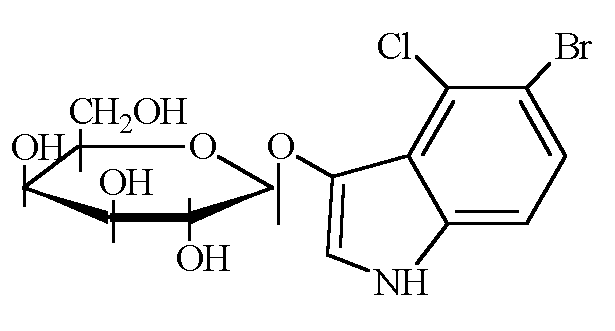
Figure 2. Chemical structure of X-gal,
substrate for β-galactosidase.
Galactose is on the left and the insoluble blue portion that is cut off is on
the right.
Perhaps the most common use of lacZ is for blue/white selection when cloning a DNA insert into a plasmid (figure 3). If the plasmid contains a version of lacZ with a polylinker, then you can determine visually whether you have cloned the insert or not. If the insert is ligated into the polylinker, then the lacZ gene will be disrupted and the colony will be white. If no insert is incorporated into the plasmid, the lacZ gene is left intact and it is able to produce β-galactosidase and the colon will turn blue. Notice in how some colonies are dark blue, some are light blue and others are pure white. You can tell that this system is not 100% error free. In fact, if the insert is a multiple of three base pairs in size, it might be possible to create an inframe insertion and still produce blue colonies. Nevertheless, blue/white selection is a helpful way to visually screen the success of your ligation and transformation before isolating plasmid DNA.
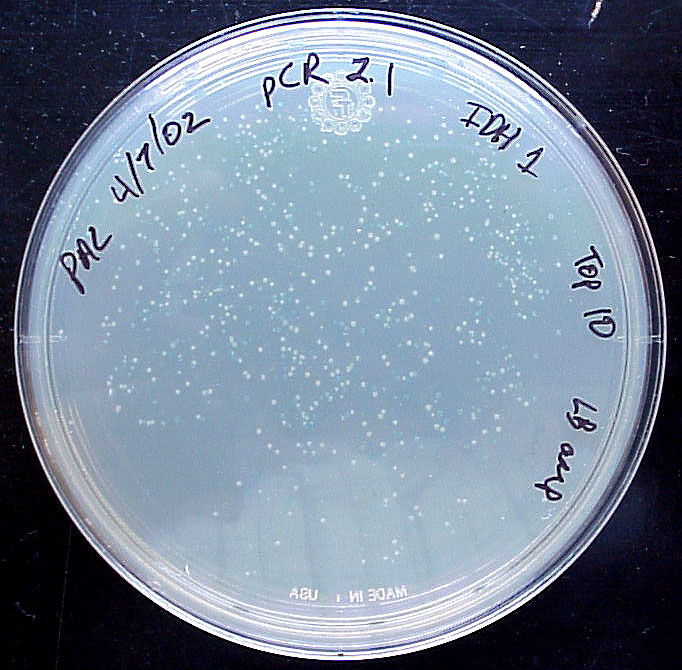
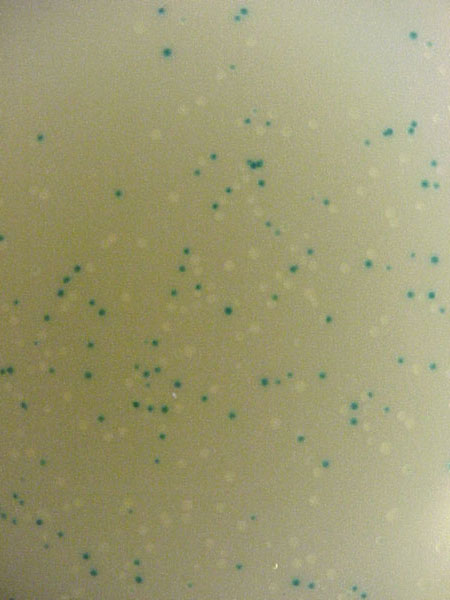
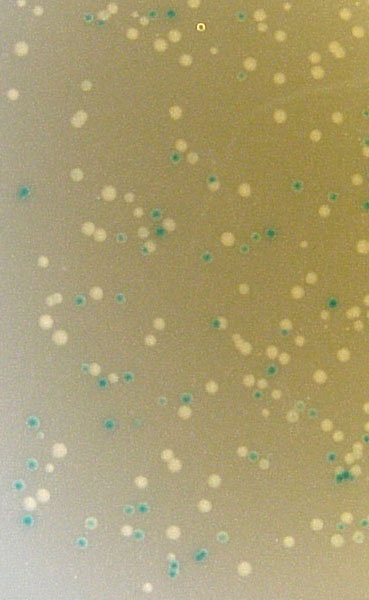
Figure 3. Blue/white selection after ligation. X-gal and IPTG were added to LB ampicillian plates prior to spreading the transformed TOP10 E. coli cells. The plasmid was pCR2.1 and the PCR product was a part of the IDH1 gene. (Top) Photograph of the entire plate. (Bottom) Photograph of same plate on white background (left) and black backgronud (right). Notice the range of blue colors and intensities.
© Copyright 2002 Department of Biology, Davidson College,
Davidson, NC 28036
Send comments, questions, and suggestions to: macampbell@davidson.edu